I. Introduction et résumé des résultats 1
1. Introduction
De nombreuses études pétrologiques sur la diversité des magmas associés aux zones de subduction ont montré que de multiples processus magmatiques tels que la fusion partielle, le mélange magmatique, la cristallisation fractionnée et l'assimilation, interviennent lors de la genèse des magmas d'arc. Quantifier les mécanismes physiques et chimiques de tels processus est difficile du fait que souvent seules les laves ou les dépôts pyroclastiques sont accessibles à l'étude. Les xénolites sont des fragments de roche entraînés par le magma dans la chambre magmatique ou pendant l'ascension ou encore à la surface lors de l'éruption. Ils sont ainsi une source d'information pétrologique et géochimique qui n'est généralement pas disponible à la surface de la Terre.
Les laves et les dépôts pyroclastiques de nombreux volcans en zone de subduction contiennent des xénolites crustaux plutoniques pour la plupart gabbroïques (Aoki, 1971, Itinome-gata, Japon; Arculus & Wills, 1980, Petites Antilles; Conrad & Kay, 1984, Adak, arc Aleutian; Grove & Donnelly-Nolan, 1986, Medicine Lake, Californie; Beard, 1986, compilation de différents sites; Yagi & Takeshita, 1987, Japon; Fichaut et al., 1989, Martinique, Petites Antilles; Beard & Borgia, 1989, Volcán Arenal, Costa Rica; Heliker, 1995, Mt. St. Helens; Hickey-Vargas et al., 1995, Volcán Calbuco, Sud Chili). L'assemblage minéralogique de ces xénolites gabbroïques se caractérise par d'importante quantité de hornblende. La présence et la composition de la hornblende (importantes teneurs en Cr2O3) dans les xénolites gabbroïques et ont été interprétées comme des évidences de cristallisation précoce de la hornblende à partir d'un magma mafique riche en eau (e.g., Conrad & Kay, 1984; Yagi & Takeshita, 1987; Beard & Borgia, 1989). Dans cette hypothèse, la cristallisation de la hornblende peut être responsable de la différenciation calco-alcaline (e.g., Yagi & Takeshita, 1987) et de la prédominance de magma andésitique (e.g., Cawthorn & O'Hara, 1976; Foden, 1983; Beard, 1986).
Cette thèse discute l'idée que les roches gabbroïques calco-alcalines (xénolites et plutons) évoluent à travers de multiples étapes de différentiation qui amènent à la précipitation tardive de hornblende et de phlogopite. Plus spécifiquement, je propose que la plupart des caractères géochimiques et pétrologiques des deux groupes de xénolites gabbroïques du Volcán San Pedro (Tatara-San Pedro Complex; TSPC; Andes chiliennes) reflètent des processus de percolation des liquides dans les zones partiellement cristallisées des chambres magmatiques. Ceux-ci impliquent la migration du liquide interstitiel évolué et de fluides aqueux, soit par compaction des cristaux, soit par convection entre la zone partiellement cristallisée et l'intérieur de la chambre. Les réactions des minéraux réfractaires précocement cristallisés (e.g., l'olivine, le spinelle chromifère, les pyroxènes et occasionnellement le plagioclase anorthitique) avec les liquides et fluides percolants, produisent d'importante quantité de hornblende (jusqu'à 50 vol.%) et de phlogopite (jusqu'à 12 vol.%) avec des compositions similaires à celles observées lors de la cristallisation précoce des magmas mafiques hydratés (e.g., hautes teneurs en Cr2O3). Les processus de migration du liquide interstitiel et les réactions, proposés pour les xénolites de San Pedro sont analogues à ceux décris pour plusieurs intrusions stratifiées tholéiitiques (e.g., Irvine, 1980, Muskox Intrusion; McBirney, 1995, Skaergaard Intrusion; Mathez, 1995, Bushveld Complex; Boudreau, 1999, Stillwater Complex) mais ont été rarement observés dans les intrusions gabbroïques calco-alcalines. Les objectifs de cette thèse sont: (1) définir les différents groupes de xénolites gabbroïques; (2) expliquer leurs multiples étapes de différentiation; (3) utiliser les zonations des plagioclases pour mettre en évidence ces mécanismes de différentiation; et (4) déterminer l'origine des compositions inhabituelles de certaines phlogopites.
1.1. Méthodes et organisation de la thèse
La thèse s'organise en quatre chapitres distincts: le Chapitre I est une introduction et un résumé des résultats. Le Chapitre II rapporte les résultats des détails texturaux, minéralogiques, et géochimiques des xénolites. Il est basé sur des analyses de microsonde électronique de tous les minéraux ainsi que sur l'analyse sur roche totale des éléments majeurs et traces et des isotopes du Sr et Ar. Dans ce chapitre, deux grands groupes de xénolites sont distingués et leur séquence de cristallisation sera discutée, incluant les évidences de migration de liquide et de fluide. Le Chapitre III rapporte les détails texturaux ainsi que les compositions en éléments majeurs et traces des plagioclases zonés d'un sous-groupe de xénolites. Cette étude est basée sur l'imagerie "Normaski Differential Interference Contrast" et sur les analyses par microsonde électronique et ionique. L'évidence des processus de migration de liquide proposé dans le Chapitre II est développée en recalculant la composition du liquide en équilibre avec le plagioclase. Le Chapitre IV porte sur l'origine des phlogopites riches en sodium présentes dans les xénolites. Pour ceci, des analyses par microsonde électronique et des images aux rayons X, ont été effectuées. Le détail des techniques analytiques est reporté sous forme d'annexe à la fin de chaque chapitre.
2. Contexte géologique et travaux antérieurs
Le complexe quaternaire Tatara-San Pedro (TSPC) se situe dans la zone volcanique des Andes, à 36°S, 71°51'W. Le TSPC (~55 km3) consiste en huit volcans, actifs durant le dernier million d'année. Bien que la plupart du TSPC ait été fortement érodé par les glaciers, les centres éruptifs des trois plus jeunes volcans sont encore préservés: Volcán Pellado, (3213 m; 188-83 ka), Volcán Tatara (3224 m; 130-105 ka), et Volcán San Pedro Holocène (3621 m) (Fig. 1). Le TSPC est le sujet d'études géologiques détaillées dont la cartographie et la stratigraphie (Dungan et al., 1992; Singer et al., 1997; Dungan et al. 1999), le paléomagnétisme (Brown et al., 1994; Pickens & Brown, 1994), et la géochronologie (Singer & Pringle, 1996; Singer et al., 1997; Singer et al., 1998) de l'édifice volcanique et de son soubassement (Nelson et al., 1999). Le TSPC est constitué principalement de laves basaltiques à rhyolitiques, avec des andésites basaltiques prédominantes (~80 vol.%). Les études pétrogénétiques antérieures (Davidson et al., 1987; Davidson et al., 1988; Ferguson et al., 1992; Singer et al., 1995; Feeley & Dungan, 1996; Feeley et al., 1998) ont montré que de nombreuses et complexes histoires magmatiques, incluant l'assimilation de croûte la supérieure et inférieure, le mélange magmatique et le mélange mécanique, ainsi que la cristallisation fractionnée, ont été enregistrées par les laves du TSPC. De petits et rares xénolites ont été trouvés dans les laves de Pellado et dans d'autres laves plus anciennes. Les plus grands (<50 cm) et les plus abondants sont présents dans une des laves du Volcán San Pedro, sujet de cette étude.
Deux plutons datés à 6.2-6.4 Ma (Nelson et al., 1999) sont présents dans le socle du complexe volcanique composé essentiellement de roches métavolcaniques. Le pluton de Risco Bayo, dans la partie nord du complexe, est formé de roches gabbroïques à leucogranitiques à enclaves (Fig. 2). Le pluton de Huemul affleure sous le flanc sud et nord du complexe et est composé de leucogranites. Les pressions estimées avec le géobaromètre Al-hornblende indiquent que les plutons se sont mis en place à 4-5 km (1-1.4 kbar; Davidson & Nelson, 1994).
2.1 Volcán San Pedro et xénolites
L'activité volcanique du Volcán San Pedro (1.5 km3) comprend deux phases: une phase de formation du cône avec des laves andésitiques et dacitiques et une phase plus jeune qui postdate l'effondrement du flan est, accompagnée d'une éruption explosive produisant des dépôts pyroclastiques dacitiques (Singer & Dungan, 1992). Cet ensemble est suivi par une séquence de laves qui enregistrent l'éruption d'une chambre magmatique fortement zonée: (1) 0.2 km3 de dacite à hornblende-biotite contenant d'abondants xénolites mafiques (jusqu'à 45 cm de diamètre) et des inclusions mafiques trempées (IMT). (2) 0.5 km3 de dacite à 2 pyroxènes avec d'abondantes IMT, et (3) 0.1 km3 d'andésites avec de rares IMT. La dernière activité volcanique consiste en 0.2 km3 d'andésites basaltiques qui reforment le cône sommital (Fig. 2). La majorité des xénolites sont gabbroïques (22 échantillons), mais quelques granites et roches métamorphiques ont également été échantillonnés. Les xénolites de petite taille sont arrondis ou sub-arrondis, tandis que les larges fragments sont anguleux (Fig. 2). Les xénolites sont présents de façon homogène dans la coulée, et représentent un volume inférieur à 10 %. Le fait que les xénolites soient exclusivement présents dans la première coulée qui a suivi l'éruption explosive du à l'effondrement du flanc est du Volcán San Pedro, suggère qu'ils sont les fragments des conduits ou des parties supérieures de la bordure de la chambre magmatique qui ont été arrachés lors de l'éruption (mécanismes similaires à ceux de l'éruption du Mt. St. Helens, le 18 mai 1980; Hekiler, 1995).
 Figure 1. Carte géologique simplifiée du complexe Tatara-San Pedro (TSPC).
Figure modifiée d'après Singer et al. (1997).
Figure 1. Carte géologique simplifiée du complexe Tatara-San Pedro (TSPC).
Figure modifiée d'après Singer et al. (1997).
Les principaux centres volcaniques peuvent être distingués: Volcán Tatara, Volcán San Pedro. Les xénolites ont été trouvés dans une des coulées dacitiques du Volcán San Pedro.
 Figure 2 A-C. Photos du Volcán San Pedro.
Figure 2 A-C. Photos du Volcán San Pedro.
A. Regardant vers le nord, vue du flanc sud du Volcán San Pedro. La coulée de lave, dans laquelle les xénolites sont présents, est marquée d'une flèche. Nous pouvons également distinguer l'affleurement de leucogranite du pluton Huemul (blanc) sur la gauche de la photo, recouvert par des roches métamorphiques et les laves du Volcán Tatara. L'escarpement du flanc effondré du volcan, qui a induit l'éruption est également visible. B. Nombreux xénolites angulaires à sub-arrondis dans la lave dacitique du Volcán San Pedro. C. Large xénolite gabbroïque (40 cm de diamètre).
3. Résumé des caractéristiques texturales, minéralogiques et géochimiques
La plupart des échantillons sont des leuconorites à clinopyroxène et hornblende, avec quelques norites à hornblende et quelques norites à olivine. Tous les échantillons ne montrent aucune altération et ne possèdent pas de minéraux secondaires hydrothermaux. Sur la base d'observations texturales et de l'abondance modale, j'ai divisé les xénolites en deux groupes principaux:
-
Groupe I: il est formé de norites à olivine-hornblende et de mélanorites, partiellement cristallisées (3 échantillons). Celles-ci se caractérisent par la présence d'un verre rhyolitique résiduel et sont probablement co-magmatiques aux magmas de San Pedro.
-
Groupe II: il consiste en un ensemble de xénolites à texture sub-solidus (pyroxènes présentant des lamelles d'exsolution) (19 échantillons). Cet ensemble a été subdivisé en deux sous-groupes: a) un Groupe IICL, composé essentiellement de leuconorites à clinopyroxène avec une grande proportion de plagioclase (> 50 vol.%), peu d'olivine (<10 vol.%) et des proportions variables de hornblende (< 40 vol.%); et b) un Groupe IIHN, composé de norites à hornblende avec une majorité de hornblende (> 30 vol.%), peu de plagioclase (< 35 vol.%) et une quantité d'olivine significative (> 10 vol.%). Les résultats des analyses 40Ar/39Ar suggèrent que ces xénolites ont au moins 1 Ma et représentent ainsi des fragments du socle plutonique pré-Quaternaire du volcan. Dans le chapitre suivant, je présente et discute les principaux caractères texturaux, minéralogiques et géochimiques des différents groupes de xénolites. Afin de montrer si la composition des xénolites a été affectée par des processus d'accumulation de minéraux ou par des processus de migration du liquide interstitiel (e.g., Irvine, 1980), je comparerai la composition des xénolites à la composition moyenne de dix basaltes de TSPC, en considérant qu'ils représentent la composition d'un liquide mafique.
3.1 Xénolites du Groupe I: norites à hornblende et olivine
La principale caractéristique pétrographique de ces échantillons est la présence de textures de réaction: l'olivine est typiquement résorbée, xénomorphe et entourée de hornblende, d'orthopyroxène et de phlogopite, et est plus rarement en contact avec le verre. Les rares clinopyroxènes sont également résorbés, xénomorphes et sont présents seulement à l'intérieur des hornblendes. Le spinelle chromifère est présent en inclusion dans l'olivine, l'orthopyroxène, la horblende et dans le coeur du plagioclase. Par contre, l'orthopyroxène, la hornblende, le plagioclase, la phlogopite, et l'apatite sont euhedrals. A partir des critères texturaux, je propose que les réactions entre olivine + spinelle chromifère + clinopyroxène et un liquide évolué, ont produit l'assemblage hornblende + orthopyroxène + phlogopite avec un fort "mg-number" et de hautes teneurs en Cr2O3 (Table 1). Plusieurs caractéristiques pétrologiques suggèrent que ces réactions ne sont pas simplement dues à une cristallisation en système fermé: (1) les réactions entre le clinopyroxène ou l'olivine et le liquide produisant la hornblende, et entre l'olivine et le liquide produisant l'orthopyroxène, ont été observées lors d'expériences effectuées avec des basaltes et andésites à faible pression (<3 kbar) (e.g., Sisson & Grove, 1993; Grove et al., 1997; Moore & Carmichael, 1998; Barclay & Carmichael, in prep.). Cependant, dans aucune de ces expériences, la co-cristallisation de la hornblende et de l'orthopyroxène (et la phlogopite) a été observée. La phlogopite n'est présente dans aucune expérimentation, même avec une cristallinité allant jusqu'à 90 % (Kawamoto, 1996). (2) Les valeurs du rapport Na/Ca des hornblendes sont élevées (pour des teneurs en Al similaires) comparées à celles des hornblendes des xénolites gabbroïques des zones de subduction et des hornblendes des expériences effectuées sur des magmas mafiques à intermédiaires à faible pression (<3 kbar). Nous proposons que ces fortes valeurs du rapport Na/Ca des hornblendes reflètent les rapports élevés Na/Ca du liquide (e.g., les dacites de San Pedro ont un rapport Na/Ca d'environ 1,2). (3) Les valeurs du rapport Na/K des phlogopites sont plus hautes que celles reportées dans les expériences à faible pression (< 3 kbar) ou que celles des autres plutons ou xénolites gabbroïques. Ceci suggère que le liquide présent lors de la cristallisation des phlogopites était riche en K2O et H2O. (4) Le plagioclase montre un abrupt changement de composition de An85-70 au coeur à An45-20 en bordure. Ceci est difficile à expliquer lors d'une cristallisation en système fermé (e.g., Brophy et al., 1996). (5) La coexistence d'olivine forstéritique et de verre rhyolitique est atypique dans les système fermé, toutefois, quelques exemples ont été décris dans des systèmes ou un mélange mécanique entre magmas felsiques et mafiques à lieu (e.g., Feeley & Dungan, 1996).
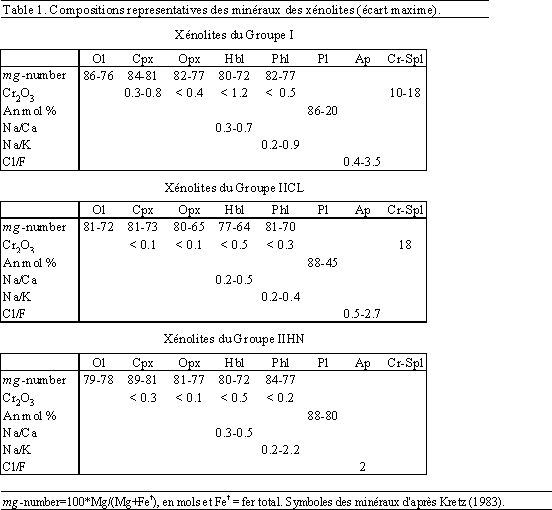 Table 1. Compositions représentatives des minéraux des xénolites (écart maximal)
Table 1. Compositions représentatives des minéraux des xénolites (écart maximal)
Afin d'expliquer toutes ces observations, je propose un scénario ou les xénolites étaient formés d'un réseau cristallin composé d'olivine, de spinelle chromifère, de clinopyroxène et de plagioclase anorthitique avec un liquide mafique remplissant les interstices. Le liquide mafique a été remplacé par un liquide plus évolué qui a réagit avec les minéraux mafiques et engendre la cristallisation de la hornblende, de l'orthopyroxène et de la phlogopite. Ceci est suivi par la cristallisation des bordures albitiques des plagioclases. Le processus de migration du liquide est probablement régit par le contraste de densité entre le liquide mafique inter-cumulus et le liquide plus évolué et plus léger qui le remplace (i.e., convection compositionnelle). Un tel processus a été reproduit en laboratoire dans des expériences de cristallisation de sels (e.g., Chen & Turner, 1980; Kerr & Tait, 1986; Tait & Jaupart, 1992).
3.2. Xénolites du Groupe IICL: leuconorites à clinopyroxène
La majorité de ces échantillons montre une texture en mosaïque, sériée, avec parfois une orientation préférentielle des plagioclases. Les clino- et orthopyroxènes sont xénomorphes à hypidiomorphes, avec des lamelles d'exsolution et sont souvent en amas interstitiels entre des cristaux de plagioclase. La hornblende et la phlogopite sont xénomorphes à hypidiomorphes, typiquement poecilitiques et entourent les minéraux résorbés et xénomorphes de pyroxène, d'olivine et de plagioclase. La hornblende et la phlogopite sont également présentes en remplissage de microfractures sub-linéaires. Les oxydes Fe-Ti sont automorphes et présents en inclusion dans les pyroxènes, mais dans de nombreux échantillons, ils sont poecilitiques et entourent le plagioclase, les pyroxènes et occasionnellement la hornblende et la phlogopite. L'apatite, automorphe, est présente entre les limites de grain des cristaux de plagioclase ou en inclusion dans la phlogopite. Contrairement au Groupe I de xénolites, de nombreux échantillons montrent des textures de rééquilibration sub-solidus le long des limites de grain des cristaux de plagioclase et entre le plagioclase et les pyroxènes (i.e., angle dièdre des limites de grain constant; Hunter, 1987). Le plagioclase montre des macles déformées, des microfissures, des limites de grain dentelées, et occasionnellement des recristallisations dynamiques de sous-grains. De plus, des microfractures discontinues (<0.5 mm de large) remplies d'oxydes, de hornblende et de phlogopite (et fréquemment d'orthopyroxène) sont présentes dans de nombreux échantillons. De telles microfractures recoupent tous les minéraux à l'exception des hornblendes poecilitiques et des phlogopites. Lorsque les microfractures intersectent le contact pyroxène-plagioclase, les deux minéraux sont résorbés, xénomorphes et sont entourés d'une bordure de hornblende ou de phlogopite. Ainsi, je propose que les microfractures étaient remplies d'un liquide ou d'un fluide aqueux qui a réagit avec les pyroxènes et le plagioclase afin de produire les minéraux hydratés.
 Table 2. Variations compositionnelles des xénolites et basaltes du TSPC.
Table 2. Variations compositionnelles des xénolites et basaltes du TSPC.
Les teneurs en éléments majeurs de ce groupe de xénolites sont similaires à celles observées dans les basaltes riches en aluminium, typiques des zones de subduction. Comparés aux basaltes du TSPC, les xénolites ont de faibles concentrations en éléments traces incompatibles (e.g. Zr, Ce, Rb) et des teneurs similaires en éléments compatibles (e.g., Sr, Ni) (Table 2). Celles-ci ne peuvent pas s'expliquer par l'accumulation des minéraux comme l'olivine et le plagioclase et suggèrent plutôt que ces xénolites ont perdu du liquide interstitiel évolué. L'étendue du rapport des éléments incompatibles (e.g., P/Zr) suggère que la perte du liquide s'est effectuée avant et après la cristallisation de l'apatite. Les lattes de plagioclase déformées et les microfractures remplies essentiellement par la hornblende et la phlogopite sont des évidences texturales et minéralogiques qui indiquent que l'expulsion du liquide interstitiel évolué s'est faite par un processus de compaction des cristaux. Le rapport des éléments incompatibles prenant en compte K et Rb montre des valeurs très disparates (e.g., Rb/Y). La mobilisation du K et Rb par rapport aux autres éléments incompatibles (e.g., Zr, Y) peut s'expliquer au moyen d'une phase fluide aqueuse. Le coefficient de partage du K et Rb entre un fluide et un liquide est beaucoup plus grand que celui de Y et Zr (e.g., Keppler, 1996). Par conséquent, les fortes teneurs en Rb et K2O et le rapport élevé Rb/Y (ou faible P/Rb) de certains xénolites peuvent être expliqués par l'arrivée d'une phase aqueuse (sous la forme de bulles) qui est dissoute dans le liquide restant. Le fluide a pu migrer le long des limites de grain des cristaux ou à travers les microfractures (e.g., Shinohara & Kazahaya, 1995). De plus, le haut rapport Cl/F de certaines apatites appuie l'idée que certains xénolites ont été affectés par l'arrivée d'un fluide aqueux. Les analyses d'isotopes stables disponibles (d18O) suggèrent que les xénolites n'ont pas été affectés par une circulation d'eau hydrothermale. Le processus décrit ci-dessus implique donc des fluides magmatiques.
3.3. Xénolites du Groupe IIHN: norites à hornblende
Les échantillons de ce groupe montrent des textures hétérogènes avec de larges hornblendes xénomorphes poecilitiques (> 1cm) qui entourent les cristaux xénomorphes et résorbés d'olivine, de clinopyroxène, d'orthopyroxène, de plagioclase, de spinelle chromifère et d'oxydes Fe-Ti. La phlogopite est également présente sous forme de cristaux poecilitiques qui entourent les cristaux résorbés et xénomorphes d'olivine, d'orthopyroxène et de plagioclase. De rares apatites sont présentes aux limites de grain des plagioclases. Comme pour les xénolites du Groupe I, j'interprète les cristaux xénomorphes et résorbés d'olivine, de pyroxènes et de plagioclase à l'intérieur de la hornblende et de la phlogopite, comme une évidence de réaction entre les minéraux anhydres et un liquide riche en eau. Il est important de noter que le plagioclase et l'orthopyroxène dans la hornblende poecilitique sont déformés (macles courbées du plagioclase et extinction irrégulière de l'orthopyroxène). Les cristaux poecilitiques de hornblende et de phlogopite ne montrent pas d'évidence claire de déformation texturale. Ainsi, je propose que la déformation a eu lieu avant la cristallisation de la hornblende et de la phlogopite.
La composition de la roche totale de ces xénolites montre de faibles teneurs en éléments incompatibles (e.g., Zr, Ce) et de fortes teneurs en Ni comparées aux basaltes TSPC (Table 2). Etant donné que la cristallisation de la hornblende se produit par réaction entre l'olivine, le plagioclase et le liquide, il est difficile d'établir, à partir des abondances modales, si les faibles concentrations en éléments incompatibles de ces xénolites sont dues uniquement à une accumulation de plagioclase et d'olivine, ou si la perte du liquide est également importante. Les rapports d'éléments incompatibles comme P/Zr et P/Y sont comparables à ceux des basaltes TSPC et suggèrent ainsi que si la migration d'un liquide interstitiel s'est produite, elle a eu lieu avant la cristallisation de l'apatite. Par contre, les rapports Rb/Y et K/Y sont plus haut que ceux observés dans les basaltes du TSPC. Utilisant les même arguments présentés pour les xénolites du Groupe IICL, je suggère qu'un fluide aqueux a percolé. Je propose ainsi, que le liquide interstitiel s'est échappé du réseau cristallin, et qu'un fluide s'est dissout dans le liquide résiduel, l'enrichissant en éléments volatils, incluant K, Rb et H2O. Ceci à produit une réaction entre les minéraux et le liquide, formant la hornblende et la phogopite avec de forts "mg-numbers" et de fortes teneurs en Cr2O3. De telles réactions sont probablement responsables des hauts rapports Na/Ca de la hornblende et Na/K de la phlogopite (Table 1). L'arrivée d'un fluide et les réactions proposées ici, peuvent être analogues aux processus décris par Boudreau (1999) pour "olivine-bearing Zone I" du Complexe Stillwater (Montana). De plus, le haut rapport Cl/F de certaines apatites supporte l'idée que les xénolites ont été affectés par un fluide. Les analyses d'isotopes stables disponibles (d18O et dD) suggèrent que le fluide aqueux est magmatique et non météoritique.
Conclusions
L'étude pétrographique et géochimique des deux groupes de xénolites gabbroïques du complexe de Tatara-San Pedro a montré que ces xénolites ont enregistré plusieurs épisodes de cristallisation liés à la migration de liquide. Les réactions des minéraux réfractaires (spinelle chromifère, olivine, pyroxènes et plagioclase) avec les liquides évolués et les fluides percolants, ont produit la cristallisation de la hornblende et de la phlogopite. Nous suggérons que la grande abondance de ces minéraux hydratés dans les gabbros calco-alcalins par rapport aux basaltes ou andésites basaltiques peut s'expliquer par une interaction entre des liquides évolués et les minéraux précocement cristallisés. Le résultat des calculs de dynamique des fluides simples suggère que la migration de ces liquides par convection compositionnelle à l'intérieur des zones partiellement cristallisées des chambres magmatiques calco-alcalines est un mécanisme de différentiation réalisable.
4. Texture et zonation en élément majeur et traces des plagioclases
Dans cette partie, j'utilise la texture et la zonation en éléments majeurs et traces des plagioclases, avec la composition du verre interstitiel pour tester l'hypothèse que la séquence de cristallisation du Groupe de xénolites I consiste en:
(1) la cristallisation de l'olivine (± spinelle chromifère), suivie par la cristallisation du clinopyroxène et du plagioclase anorthitique (An86-An70), (2) le remplacement du liquide interstitiel mafique par un liquide plus évolué (e.g., dacitique), et (3) la réaction du liquide dacitique avec olivine + spinelle chromifère + clinopyroxène, pour former orthopyroxène + hornblende + phlogopite, suivie par la cristallisation du plagioclase pauvre en anorthite (e.g. An45-An6) et de l'apatite.
4.1. Texture et zonation en éléments majeurs des plagioclases
Des traverses de 42 cristaux de plagioclase allant du centre à la bordure (échantillons Hx14n and Hx14b) ont été effectuées avec la microsonde électronique (environ 2500 analyses). Dans la discussion suivante, le coeur des plagioclases correspond à des compositions comprises entre An86 et An70 mol%, la zone de transition entre le coeur et la bordure correspond à des compositions comprises entre An70 et An45, et la bordure correspond à des compositions < An45.
Les cristaux de plagioclase des deux échantillons montrent des zonations en éléments majeurs et des textures comparables, avec l'exception que les plagioclases de l'échantillon Hx14b montrent plus de surfaces de dissolution et une zonation oscillatoire plus développée que dans l'échantillon Hx14n. Les cristaux de plagioclase qui ne sont pas inclus dans l'orthopyroxène, la hornblende, ou la phlogopite montrent un coeur An86 à An70 avec une zonation normale. Le coeur des plagioclases est entouré d'une bordure à zonation normale de An45 à An20. Le passage entre le coeur et la bordure se fait par un abrupt (en moins de 100 µm) changement de composition de 30 à 35 mol% d'anorthite (Fig. 3). Les cristaux de plagioclase en inclusion dans l'orthopyroxène, la hornblende, ou la phlogopite ont les mêmes compositions et présentent une zonation normale de An86 à An40, ce qui indique que ces trois minéraux mafiques ont co-cristallisé.
4.2. Zonation en éléments traces des plagioclases
Des traverses du centre au bord des plagioclases par microsonde ionique ont été effectuées sur neuf cristaux de plagioclase représentatifs de l'ensemble des échantillons. Au total, 90 analyses ont été effectuées et les éléments suivants ont été analysés: Ca, Mg, Fe, Ti, K, Rb, Sr, Ba, La, Ce, Y, et Li.
Les concentrations en Ti, Sr, Ba, La, Ce, et Li des coeurs des plagioclases augmentent en allant vers la zone de transition. Les teneurs en Fe restent plus ou moins constantes, tandis que les teneurs en K augmentent dans certains cristaux et diminuent dans d'autres. Les concentrations en Mg diminuent toujours. Les concentrations en Rb et Y ne définissent pas réellement un enrichissement ou un appauvrissement, probablement du fait de leurs larges erreurs analytiques (25-30 %). De grandes et abruptes variations de concentration en Fe, Ti, Sr, Ba, K, La, Ce, et Y sont présentes à la transition coeur-bordure. Les bordures ont des concentrations plus élevées en Sr, Ba, K, La, Ce et plus faibles en Fe, Ti, et Y que les coeurs (Fig. 3). Les concentrations en Mg, Fe, Ti, La, et Ce restent plus ou moins contantes de l'intérieur vers l'extérieur de la bordure de tous les cristaux. Les concentrations en Sr, Ba et K ont tendance à augmenter vers la partie extérieure de la bordure.
4.3. Liquides en équilibre avec les plagioclases
Dans cette partie, je calcule la composition des liquides en équilibre avec les plagioclases en utilisant les équations de Blundy & Wood (1991; équations #18 and #19) pour Sr et Ba, et les équations de Bindeman et al., (1998) pour Mg, Ti, Fe, K, La, et Ce. On observe que:
-
Les liquides en équilibre avec les coeurs des plagioclases montrent un enrichissement en TiO2, Sr, Ba, K2O, La, et Ce et un appauvrissement en MgO, qui sont comparables à l'évolution des laves basaltiques à basaltiques-andésitiques du Volcán San Pedro, et qui s'expliquent par la cristallisation de olivine + clinopyroxène + plagioclase anorthitique.
-
Les liquides en équilibre avec les zones de transition coeur-bordure des plagioclases montrent de grandes variations de concentration, comparables à l'évolution des laves basaltiques à andésitiques du Volcán San Pedro.
-
Les liquides en équilibre avec les bordures des plagioclases ont de plus faibles concentrations en TiO2, FeO* et Sr, et de plus fortes concentrations en K, Ba, et La que le liquide en équilibre avec le coeur des plagioclases. De telles différences de composition peuvent être expliquées par la cristallisation de la hornblende (± orthopyroxène et phlogopite).
-
Les liquides en équilibre avec les bordures des plagioclases montrent une diminution en Sr et Ba et une augmentation en K/Ba et K2O, tandis que les concentrations en TiO2, MgO, La, et Ce restent plus ou moins constantes. Cette tendance n'est pas représentée dans les laves de San Pedro et peut s'expliquer par la cristallisation d'un assemblage minéralogique dominé par la phlogopite et le plagioclase albitique (e.g., <An40) et montre ainsi l'évolution d'un liquide de composition dacitique à rhyolitique. Le fait que les concentrations en La et Ce restent plus ou moins constantes, pourrai s'expliquer par la cristallisation de l'apatite (présente dans le verre) ou par les hauts coefficients de partage de La et Ce reportés pour les biotites (e.g., Nash & Crecraft, 1985).
Bien que les liquides en équilibre avec les bordures des plagioclases montrent une évolution en accord avec le fractionnement d'un liquide dacitique à rhyolitique, les concentrations absolues calculées pour Ba et K2O sont environ 50 % plus basses et celles de La et Ce sont 50 % plus hautes, comparées aux compositions du verre interstitiel rhyolitique et aux laves et plutons dacitiques et rhyolitiques du TSPC. Après avoir considéré les différents facteurs (incluant la cristallisation en système fermé et la cinétique de cristallisation) pouvant être responsables de la différence entre les compositions calculées et celles des liquides évolués du TSPC, deux semblent possibles: (1) les coefficients de partage obtenus à partir des équations empiriques de Blundy & Wood (1991) et Bindeman et al. (1998) ne sont peut être pas valables pour les compositions évoluées des liquides et plagioclases considérées ici, et (2) étant donné que le K, Rb et Ba sont fortement compatibles dans la phlogopite, il semble possible que les faibles concentrations en ces éléments calculées pour le liquide percolant résultent de la cristallisation de la phlogopite. L'arrêt de la cristallisation de la phlogopite et la poursuite du processus de migration du liquide pourrai expliquer que la composition du verre interstitiel analysé ne reflète pas une cristallisation prolongée mais la composition du liquide migrant.
 Figure 3. Profils de composition en anorthite et en éléments traces des cristaux de plagioclase de l'échantillon Hx14n.
Figure 3. Profils de composition en anorthite et en éléments traces des cristaux de plagioclase de l'échantillon Hx14n.
Les bandes grises indiquent la zone de transition entre le coeur et la bordure. Notez que les éléments traces suivent l'abrupt changement en éléments majeurs suggérant un changement majeur dans la composition du liquide interstitiel. La taille des symboles est égale ou plus grande à la précision de 2-s de tous les éléments.
Conclusions
La zonation texturale et de composition en éléments majeurs et traces des plagioclases, combinée avec la composition du verre interstitiel d'une suite de xénolites gabbroïques, a permis de retracer les complexes processus de différentiation qui peuvent avoir lieu dans les zones partiellement cristallisées des chambres magmatiques. Les compositions des liquides recalculées en équilibre avec les plagioclases indiquent qu'après une première étape de différentiation d'un liquide basaltique à basaltique-andésitique, le liquide interstitiel a évolué brusquement vers des compositions dacitiques. Ceci peut être expliqué par la migration du liquide à travers le réseau cristallin. Le liquide mafique interstitiel a été remplacé par un liquide dacitique qui a réagit avec les minéraux préexistants (olivine, clinopyroxène et spinelle chromifère), et a formé l'orthopyroxène, la hornblende, et la phlogopite. Pendant et après ces processus, le liquide interstitiel a évolué vers des compositions rhyolitiques. Cependant, pour les compositions des plagioclases (e.g., < An40) ou des liquides évolués, les compositions recalculés sont différentes de celles du verre rhyolitique interstitiel des xénolites et des laves et plutons dacitiques du TSPC. Ceci peut être du au fait de la complexité des processus de migration des liquides et des réactions qui se produisent dans les zones partiellement cristallisées des chambres magmatiques, ou au fait de l'incertitude des coefficients de partage plagioclase-liquide des magmas siliciques.
5. Origine des phlogopites riches en Na
La présence et la composition de la phlogopite (jusqu'à 5 % pds (poids) de Na2O) dans les trois groupes de xénolites gabbroïques sont des caractères inhabituels des roches gabbroïques calco-alcalines. Jusqu'à présent, la phlogopite avec de fortes teneurs en Na2O a été seulement décrite sous forme d'inclusion mono- ou polyminérale dans les intrusions stratifiées (e.g., Stillwater complex, Talkington et al., 1986; Laouni intrusion, Lorand & Cottin, 1987) et dans les complexes ophiolitiques (Hongguleleng ophiolite, Peng et al., 1995). Dans ces contextes, la phlogopite riche en Na est communément associée avec la phlogopite, l'olivine, les pyroxènes, l'albite, les feldspathoïdes, et l'amphibole riche en Na.
Dans le Groupe I de xénolites, la phlogopite représente jusqu'à 8 vol.%. Elle est présente dans le verre interstitiel sous forme de cristaux xénomorphes à hypidiomorphes et entoure les cristaux résorbés d'olivine et de spinelle chromifère. Les phlogopites ont des "mg-numbers" élevés et de fortes teneurs en Cr2O3 (Table 2). Les teneurs en Na2O varient de 1,5 à 3,5 % pds, et le rapport Na/K de 0,2 à 0,9. L'occupation du site A varie de 0,9 à 1, ce qui différencie ces phlogopites des wonesites qui ont des couches interstratifiées incomplètes (Spear et al., 1981).
Dans le Groupe IICL de xénolites, la phlogopite (jusqu'à 15 vol.%) se présente sous la forme de petits cristaux poecilitiques (< 1 mm) entourant les cristaux résorbés de plagioclase, d'orthopyroxène et d'oxydes Fe-Ti. La phlogopite est également présente en remplissage des microfractures. Les phlogopites poecilitiques ont des "mg-numbers" qui varient de 81 à 70 et des teneurs en Cr2O3 inférieures à 0,2 % pds. Les teneurs en Na2O (1,1-2,1 % pds) et le rapport Na/K (0,2-0,4) sont plus faibles que ceux des phlogopites du Groupe de xénolites I. Les phlogopites remplissant les microfractures ont de plus faibles "mg-numbers", de plus faibles teneurs en Cr2O3 (< 0,15 % pds) et Na2O (0,7-1,3 % pds), et un plus faible rapport Na/K (~ 0.2) que les phlogopites poecilitiques. Je propose que les plus hauts "mg-numbers" et les plus fortes teneurs en Cr2O3 des phlogopites poecilitiques sont le résultat de réactions entre les minéraux mafiques (e.g., l'olivine, le spinelle chromifère, et les pyroxènes) et un liquide évolué qui a pu migrer à travers les microfractures.
Dans le Groupe IIHN de xénolites, la phlogopite représente 4 vol.% et est généralement hypidiomorphe à xénomorphe. Elle est en inclusion dans les cristaux de hornblende, entoure les cristaux résorbés d'olivine, de spinelle chromifère et d'orthopyroxène. Contrairement au Groupe I, elle est également présente autour du plagioclase résorbé. Les phlogopites ont de forts "mg-numbers" variant de 84 à 77, et des teneurs en Cr2O3 allant jusqu'à 0,2 % pds. La plupart des phlogopites ont des concentrations en Na2O comprises entre 1 et 2,5 % pds, à l'exception d'un cristal poecilitique qui contient jusqu'à 5 % pds de Na2O, et un rapport Na/K allant jusqu'à 2,2. Une carte d'élément par rayons X de ce cristal de phlogopite montre que les concentrations en Na ne sont pas homogènes (Fig. 4). Le cristal se compose de bandes riches en Na (< 50 µm) qui sont parallèles aux clivages (parallèle au plan 001) et qui correspondent à des zones pauvres en K. Une traverse par microsonde électronique à travers la zone riche en Na de ce cristal montre des variations en Na2O de 2 % pds sur moins de 10 µm, suggérant que les zones riches en Na sont hétérogènes et probablement consistent en de fins (< 1-2 µm) enchevêtrements de phlogopite riche en Na et de phlogopite sensu stricto (Fig. 5).
 Figure 4. Cartes de distribution par rayons X de Na et K.
Figure 4. Cartes de distribution par rayons X de Na et K.
La distribution de Na et K forme des bandes approximativement parallèles aux clivages. La ligne de tirets marque la position de la traverse effectuée à la microsonde électronique montrée dans la Figure 5.
 Figure 5. Traverse de la zone riche en Na du cristal de phlogopite montré en Figure 4.
Figure 5. Traverse de la zone riche en Na du cristal de phlogopite montré en Figure 4.
A l'intérieur de la zone riche en Na, on observe des variations de concentration en Na2O allant jusqu'à 2 % pds suggérant que les zones riches en Na sont hétérogènes et consistent en de fins enchevêtrements de phlogopite riche en Na et de phlogopite sensu stricto.
De tels changements de composition en Na2O et K2O dans la phlogopite sont difficiles à expliquer par des changements de composition du liquide seul, et reflètent la présence d'un solvus.
Je propose que les hauts "mg-numbers", les fortes teneurs en Cr2O3 et les concentrations modérées en Na2O (2 % pds) de certaines phlogopites peuvent s'expliquer par des processus en système ouvert de migration de liquides évolués riches en eau et de réactions avec les minéraux mafiques précocement cristallisés (olivine, spinelle chromifère, et pyroxènes). Les hautes teneurs en Na2O (jusqu'à 5 % pds) et le haut rapport Na/K (2,2) des autres phlogopites semblent impliquer des réactions avec des liquides ayant un rapport Na/K invraisemblablement élevé. Ceci, plus le fait que les cristaux de phlogopite consistent en de fins enchevêtrements de phlogopite riche en Na et de phlogopite pauvre en Na, suggèrent qu'il y a un solvus entre les deux pôles de composition (Na-K) des phlogopites. La présence de phlogopite riche en sodium dans les deux groupes de xénolites, d'âge différent, suggère qu'elle peut être un minéral plus commun que ce qui a été reconnu dans les systèmes calco-alcalins.
II. Hornblende and Phlogopite-bearing gabbroic crustal xenoliths from Volcán San Pedro (36o S), Chilean andes: insights to melt (and fluid) migration and reaction processes in calc-alkaline plutons
Abstract
A late Holocene eruption of Volcán San Pedro (Tatara-San Pedro Volcanic Complex, TSPC, 36o S, Chilean Andes) brought to the surface two groups of gabbroic xenoliths. Group I comprises olivine melanorites and norites bearing interstitial residual glass and thus are potentially co-magmatic with the Holocene volcanism. Group II consists of clinopyroxene leuconorites (CL) and hornblende norites (HN). These display subsolidus and deformation textures suggesting they are fragments of the plutonic basement of the TSPC. Both groups of xenoliths show reaction relations between early-formed refractory minerals (olivine, Cr-spinel, pyroxenes, or plagioclase) and late hornblende and phlogopite. A number of petrologic features suggest that these reactions are not simply due to closed-system crystallization: (1) The Na/Ca of most hornblendes is high (0.3-0.7) compared to hornblendes from other subduction-related gabbroic xenoliths or to hornblendes from low pressure experiments of basaltic to andesitic compositions. (2) Phlogopite has extremely high Na/K (0.2-2.2) approaching those of pure sodium phlogopite. (3) Group II xenoliths display bulk-rock trace element concentrations and ratios of incompatible elements that are consistent with loss of evolved melt and fluxing by an aqueous fluid (4) Microfractures filled with hornblende and phlogopite are evidence of melt and fluid migration. (5) The halogen concentrations of apatite (high Cl/F) suggest they crystallized from fluid-enriched melts. We propose that the textures, bulk-rock, and mineral compositions of these xenoliths have been substantially modified by interactions between early-crystallized minerals and percolating evolved melts and aqueous fluids. It is argued that the abundance of hornblende in calc-alkaline gabbroic plutons and xenoliths compared to its paucity in basaltic or basaltic andesitic lavas can be explained by open-system percolative processes analogous to those documented for many layered tholeiitic intrusions.
1. Introduction
It is well established that melt and fluid migration through a partly crystalline framework is an important differentiation mechanism in tholeiitic layered intrusions. Specifically, melt and fluid migration in tholeiitic intrusions have been invoked to explain: (1) shifts in the expected crystallization sequence (e.g., Bushveld Complex, Nicholson & Mathez, 1991; Stillwater Complex, Meurer et al., 1997; Boudreau, 1999), (2) changes of the mineral proportions and compositions caused by reactions between the cumulus minerals and the percolating liquids (e.g., Muskox Intrusion, Irvine, 1980; Skaergaard Intrusion, McBirney & Sonnethal, 1990; Bushveld Complex, Mathez, 1995), and (3) depletions of incompatible elements in the lower sections and enrichments in the upper-middle sections, including the formation of ore deposits (e.g., Stillwater Complex, Boudreau & McCallum, 1992; Skaergaard Intrusion, McBirney, 1995; Bushveld Complex, Mathez 1995). Experiments and theoretical work on fluid dynamics through porous media support the idea that density-driven convection or compaction are indeed feasible differentiation mechanisms in tholeiitic systems (e.g., Chen & Turner, 1980; Huppert et al., 1986; Kerr & Tait, 1986; Martin et al., 1987; McKenzie, 1987; Tait & Jaupart, 1992; Marsh, 1995; Hort et al., 1999).
In contrast, the compositional and petrological imprints of open-system percolative processes in calc-alkaline gabbroic rocks have been rarely documented (e.g., Sawka et al., 1990; DuBray & Harlan, 1996). Because calc-alkaline magmas have higher water contents compared to tholeiitic, they have different densities, viscosities, and mineral assemblages, one would expect that the compositional and mineralogical effects of melt migration and reaction in calc-alkaline systems to be different.
In this chapter we present a detailed mineralogical, textural, and compositional study of two groups of gabbroic xenoliths from the calc-alkaline Volcán San Pedro (Tatara-San Pedro Volcanic Complex, TSPC, 36o S, Chilean Andes). These xenoliths record complex crystallization histories that involve migration of evolved interstitial liquids driven by compaction and convection, and reactions between refractory cumulus minerals (olivine, Cr-spinel, pyroxenes, and plagioclase) and percolating melts and aqueous fluids. The main effects of these interactions were to produce substantial amounts of hornblende (up to 50 vol. %) and phlogopite (up to 15 vol. %), from which we suggest that the larger proportions of these hydrous minerals in calc-alkaline gabbros compared to basalts can be explained by open-system percolative processes.
2. Geological setting and previous work
The Quaternary Tatara-San Pedro Complex (TSPC) is located on the volcanic front of the Southern Volcanic Zone (SVZ) of the Andes, at 36o S, 71o51'W (Fig. 1). The TSPC (~ 55 km3) consists of a least eight different volcanoes that were active during the last million years. Although much of the TSPC has been substantially eroded by glaciers, the vents of the three youngest volcanoes are still preserved: Volcán Pellado (3213 m; 188-83 ka), Volcán Tatara (3224 m, 105-130 ka), and the Holocene Volcán San Pedro (3621 m) (Fig. 2). The TSPC is the subject of detailed geological studies including mapping, stratigraphy and sampling (Dungan et al., 1992; Singer et al., 1997; Dungan et al., submitted), paleomagnetism (Brown et al., 1994; Pickens & Brown, 1994), and geochronology (Singer & Pringle, 1996; Singer et al., 1997; Singer et al., 1998) of the volcanic edifice and of the basement around it (Nelson et al., 1999). The TSPC consists mainly of lavas with compositions ranging from basalt to rhyolite, although basaltic andesite predominates (~ 80 vol. %). Previous petrogenetic modeling studies (Davidson et al., 1987; Davidson et al., 1988; Ferguson et al., 1992; Singer et al., 1995; Feeley & Dungan, 1996; Feeley et al., 1998) have shown that complex and diverse magmatic histories including lower and upper crustal assimilation, magma mixing and mingling, and fractional crystallization have been recorded in the lavas of the TSPC. Scarce and small (<5 cm) xenoliths have been found in some Pellado and older lavas, but the largest (< 50 cm) and most abundant xenoliths occur in one lava of Volcán San Pedro. The latter are the subject of this study.
The basement rocks of the volcanic complex consist of metavolcanic rocks that are intruded by two granitoid plutons dated at 6.2-6.4 Ma (Nelson et al., 1999). The Risco Bayo pluton crops out at the northern part of the complex, is mainly an enclave-bearing granodiorite but it ranges from gabbro to leucogranite (Fig. 2). The Huemul pluton crops beneath the southern and northern flanks of the complex and is a leucogranite. Pressure estimates with the Al-in hornblende geobarometer indicate that the plutons were emplaced at ~ 4-5 km (1-1.4 kbar; Davidson & Nelson, 1994).
2.1. Volcán San Pedro and xenoliths
Volcanic activity at Volcán San Pedro (1.5 km3) is divided (Singer & Dungan, 1992) into a cone-building phase comprising andesitic and dacitic lavas and a younger phase that post-dates a sector collapse of the eastern flank, which was accompanied by an explosive eruption that produced air-fall dacitic deposits. This was followed by a sequence of lavas that apparently records the downward tapping of a strongly zoned magma chamber. The eruptive sequence comprises: (1) 0.2 km3 of biotite-hornblende dacite containing abundant mafic xenoliths (up to 45 cm in diameter) and quenched mafic inclusions (QMI), (2) 0.5 km3 of two-pyroxene dacite with abundant QMI, and (3) 0.1 km3 of two-pyroxene andesite with rare QMI. The last volcanic activity at this cone consisted of 0.2 km3 of basaltic andesite magma that rebuilt the summit cone. The majority of the xenoliths are gabbroic (22 samples), although scarce granites (1 sample) and metamorphic rocks (1 sample) were also collected. Small xenoliths are rounded to subrounded, whereas larger fragments are angular (Fig. 3). The xenoliths are homogeneously distributed in the first and most evolved flow of the post-collapse eruptive sequence. They commonly are <10 vol. %. The fact that the xenoliths have exclusively been found in the first lava flow following the catastrophic structural failure of the east flank of Volcán San Pedro suggests that they are fragments of the conduits or upper parts of the margins of the San Pedro magma chamber that were shattered during the eruption (in a similar fashion to the May 18, 1980 Mount St. Helens eruption; Heliker, 1995).
 Figure 1. Simplified geological map of Central Chile showing the location of the Tatara-San Pedro Volcanic Complex.
Figure 1. Simplified geological map of Central Chile showing the location of the Tatara-San Pedro Volcanic Complex.
Black triangles indicate main Quaternary volcanic centres. Pz-Palaeozoic rocks, Mz-Mesozoic rocks. Grey shaded areas indicate Tertiary plutons.
Figure modified from Hildreth & Moorbath (1988) and from Dungan et al. (submitted). The location of Tertiary plutons is from Mapa Geológico de Chile (1982).
 Figure 2. Simplified geological map of the Tatara-San Pedro Volcanic Complex.
Figure 2. Simplified geological map of the Tatara-San Pedro Volcanic Complex.
Note the two plutons that crop out in the northern and southern part of the complex. The xenoliths were found in a dacite flow of Volcán San Pedro.
Figure modified from Singer et al. (1997).
 Figure 3 A-D. Field and hand speciment aspect of the xenoliths.
Figure 3 A-D. Field and hand speciment aspect of the xenoliths.
A. Numerous angular to subrounded xenoliths in the biotite-hornblende dacite lava flow of Volcán San Pedro. B. Large gabbroic xenolith (40 cm diameter). C. Olivine norite xenolith (sample Hx14n) bearing interstitial glass. Lava is on the left corner of the picture. Scale bar is 12 cm across. D. Hornblende leuconorite xenolith (sample Hx14y). Note the NW-SE trending microfractures. Brown patches are hornblende and phlogopite. Scale bar is 12 cm across.
3. Textures of the Gabroic xenoliths
The majority of the samples are clinopyroxene leuconorites and norites, with minor hornblende norites and olivine norites (Table 1). All samples lack alteration or secondary hydrothermal minerals. On the basis of textural observations and modal abundances (Fig. 4), we have divided the xenoliths into two main groups (Table 1):
-
Group I consists of partially crystallized olivine-hornblende norites and melanorites (3 samples). These are characterized by the presence of interstitial residual glass and thus are potentially co-magmatic with San Pedro magmas.
-
Group II consists of xenoliths with subsolidus and deformation textures (19 samples). These have been further subdivided into: a) Group IICL are mainly clinopyroxene leuconorites with high proportions of plagioclase (> 50 vol. %), low olivine (< 10 vol. %) and variable amounts of hornblende (< 40 vol. %), and b) Group IIHN are hornblende norites with high modal proportions of poikilitic hornblende (> 30 vol. %), low plagioclase (< 35 vol. %) and significant amounts of olivine (> 10 vol. %). These xenoliths are fragments of the pre-Quaternary the plutonic basement of the volcano. Xenoliths of Group IICL have modal compositions similar to most gabbroic xenoliths found at subduction-related volcanoes, whereas those of Groups I and IIHN are unusual (see Fig. 4).
 Figure 4. Mineral modes of the xenoliths compared to other gabbroic xenoliths from subduction-related volcanoes.
Figure 4. Mineral modes of the xenoliths compared to other gabbroic xenoliths from subduction-related volcanoes.
Group IICL xenoliths have similar modes to other xenoliths, whereas Group I and Group IIHN have less common compositions. Data sources: Mt. Pelée xenoliths (Fichaut et al., 1989), Mt. St. Helens (Heliker, 1995), Medicine Lake (Grove & Donnelly-Nolan, 1986), Calbuco (Hickey-Vargas et al., 1995) and Lesser Antilles (including quartz-bearing gabbros of Arculus & Wills, 1980).
Figure modified from Arculus & Wills (1980).
3.1. Group I. Partially crystallized olivine norites
These samples consist of olivine, orthopyroxene, hornblende, plagioclase, and phlogopite forming a medium grained (1-5 mm) crystal network with interstitial vesiculated SiO2-rich (67-72 wt %) glass filling the interstices. Glass is distributed in isolated and occasionally interconnected pockets bounded by euhedral crystal faces suggesting that it is residual from crystallization and not due to partial melting (Fig. 5). A salient petrographic feature of these samples is the presence of reaction textures: olivine is typically resorbed, anhedral and surrounded by hornblende, orthopyroxene, and phlogopite, but is also much less commonly in contact with glass (Fig. 5). Rare clinopyroxene is also resorbed, anhedral and present only within hornblende. Cr-spinel occurs as inclusions in olivine, orthopyroxene, hornblende, and in plagioclase cores. In contrast, orthopyroxene, hornblende, plagioclase, phlogopite, and apatite are commonly euhedral (Fig. 5). From textural criteria we infer reaction relations between the early-crystallized cumulus olivine and clinopyroxene and the interstitial liquid to produce post-cumulus hornblende, orthopyroxene, and phlogopite (Fig. 5 and Table 2).
 Figure 5 A-D. Photomicrographs of Group I xenoliths.
Figure 5 A-D. Photomicrographs of Group I xenoliths.
A. Sample Hx14n. Anhedral, resorbed Ol surrounded by Hbl and Opx. This suggests a reaction relation between the interstitial liquid and Ol to produce Hbl and Opx. B. Sample Hx14k. Hbl enclosing anhedral, resorbed Ol, but euhedral Pl. Note also Phl surrounding anhedral Ol.. C. Sample Hx14b. Vesiculated interstitial SiO2-rich glass (66-72 wt %) in contact with euhedral Opx, Hbl and Phl. Also note the Ol crystals (Fo82) in contact with the silica-rich glass. D. Sample Hx14b. Detail of an euhedral Phl in vesiculated residual glass. Phl is surrounding anhedral, resrorbed Ol crystals suggesting a reaction relation between the interstitial liquid and Ol to produce Phl.
3.2. Group IICL. Clinopyroxene leuconorites with subsolidus textures
The majority of these samples are characterized by a mosaic, seriate texture (Shelley, 1993), in some case with plagioclases displaying a preferred orientation. Anhedral to subhedral clino- and orthopyroxenes display exsolution lamellae in many samples and commonly occur as clusters interstitial to larger plagioclase crystals. Orthopyroxene occasionally occurs as oikocrysts including plagioclase, and in some samples is present in microfractures (see below). Olivine is commonly anhedral and contains inclusions of Cr-spinel (only present in sample Hx14e). It is commonly surrounded by rims of orthopyroxene, Fe-Ti oxide symplectites, and occasionally, by small flakes of phlogopite which we refer to as late phlogopite.
Subhedral to anhedral hornblende and phlogopite typically occur as small (< 1 mm across) crystals surrounding resorbed, anhedral pyroxenes, olivine, and plagioclase. Hornblende and phlogopite are also found filling sub-linear microfractures (Fig. 6). Radial aggregates of late phlogopite are occasionally present as the replacement products of hornblende, as reaction rims around olivine, and in some cases after orthopyroxene. Euhedral Fe-Ti oxides occur as inclusions in pyroxenes, but in many samples these are poikilitic, anhedral and they surround anhedral plagioclase, pyroxenes and occasionally hornblende and phlogopite (Fig. 6). Anhedral apatite is the only accessory mineral and it occurs between grain boundaries of plagioclase crystals or inside phlogopite.
In contrast to Group I xenoliths, most samples show subsolidus textural reequilibration along grain boundaries between plagioclase crystals and between plagioclase and pyroxenes (i.e., constant grain boundary dihedral angles between crystals; Hunter, 1987). These samples also have been variably deformed (see Table 1). Plagioclase commonly shows bent twins, microcracks, and serrated grain boundaries, and occasionally subgrain dynamic recrystallization (Fig. 6). Orthopyroxene and hornblende oikocrysts occasionally display weak uneven extinction may be related to subgrain recrystallization, whereas phlogopite oikocrysts show kink-bands. In addition, discontinuous microfractures (<0.5 mm in width) filled with Fe-Ti oxides, hornblende and phlogopite (and frequently also orthopyroxene) are present in many samples (Fig. 6). Such microfractures cut across all minerals except poikilitic hornblende and phlogopite. Where microfractures intersect pyroxene-plagioclase contacts, both minerals are resorbed, anhedral and they are mantled by a rim of hornblende or phlogopite (Fig. 6). From this textural relation we infer that the microfractures hosted melts or aqueous fluids that reacted with pyroxenes and plagioclase to produce the hydrous minerals (Table 2).
Minor amounts of glass (<6.5 vol. %) are present in some of these samples. Glass occurs exclusively along resorbed grain boundaries of plagioclase crystals, plagioclase-orthopyroxene, and plagioclase-hornblende, indicating that it is the result of partial melting and not residual from crystallization as is the case of Group I xenoliths. Small euhedral crystals of orthopyroxene, clinopyroxene and plagioclase are present in the glass.
 Figure 6 A-F. Photomicrographs of Group IICL xenoliths.
Figure 6 A-F. Photomicrographs of Group IICL xenoliths.
A. Sample Hx14a. Typical leuconorite consisting of abundant Pl, Cpx, Opx and Fe-Ti oxides. B. Sample Hx14i. Poikilitic Fe-Ti oxides enclosing resorbed Pl crystals. Note Hbl crystallized on pyroxenes and the small microfracture on the right corner of the picture. C. Sample Hx14i. Note microfracture cutting across Pl and filled with Hbl and Phl. Where the microfracture intersects pyroxenes, larger Hbl crystals are present. D. Sample Hx14q. Large microfracture filled with Hbl and Phl. Note also the numerous smaller microfractures filled with Hbl and Phl. E. Sample Hx14y. Poikilitic Hbl surrounding subrounded Pl crystals. Note how Pl crystals with bent twins (black arrows) are also fractured. F. Sample Hx14y. Fractured and bent Pl crystals with microfractures filled with Hbl, Phl and in some cases Opx (center of the picture).
3.3. Group IIHN. Hornblende norites with subsolidus textures
Samples from this group are texturally heterogeneous and are characterized by large anhedral hornblende oikocrysts (>1cm) that surround anhedral, resorbed olivine, clinopyroxene, orthopyroxene, plagioclase, Cr-spinel, and Fe-Ti oxides (Fig. 7). Where hornblende is absent, the samples consist of large orthopyroxene crystals (up to 5 mm long) and a mosaic of subhedral plagioclase. Phlogopite is also present as oikocrysts that include resorbed, anhedral olivine, orthopyroxene, and plagioclase. It occasionally occurs inside hornblende oikocrysts. Rare anhedral apatite is present along plagioclase grain boundaries.
As for the previous groups of xenoliths, we interpret the anhedral and resorbed olivine, pyroxenes, and plagioclase inside hornblende and phlogopite as evidence of reaction relations between the anhydrous minerals and a water-rich melt (Table 2). It is worth noting that plagioclase and orthopyroxene inside hornblende oikocrysts are deformed (bent twins in plagioclase and uneven extinction in orthopyroxene, Fig. 7). Hornblende oikocrysts do not show clear textural evidence of deformation, whereas phlogopite shows occasionally kink bands. From these textural relations we infer that a main deformation event occurred prior to hornblende and phlogopite crystallization.
Small and discontinuous microfractures (<0.5 mm) cut through plagioclase crystals and are filled either with hornblende, phlogopite or plagioclase. Hornblende oikocrysts are not cut by the microfractures. These samples are also partially melted, with minor amounts (<0.5 vol. %) of glass occurring along resorbed grain boundaries of plagioclase crystals, plagioclase-orthopyroxene and plagioclase-hornblende.
| Table 2. Proposed reactions derived from textural observations |
| |
Orthopyroxene |
Hornblende |
Phlogopite |
| Group I.Olivine norites |
li + Ol ± Cr-Spl |
li + Ol ± Cr-Splli + Cpx ± Cr-Spl |
li + Ol ± Cr-Spl |
| Group IICL.Clinopyroxene leuconorites |
li + Ol |
li + Cpxli + Opxli + Ol + Pl ± Cr-Spl li + Cpx + Pl ± Cr-Splli + Opx + Pl ± Cr-Spl |
li + Ol ± Pl ± Cr-Spl |
| Group IIHN.Hornblende norites |
li + Ol |
li + Ol + Pl ± Cr-Spl li + Cpx + Pl ± Cr-Splli + Opx + Pl ± Cr-Spl |
li + Ol + Pl ± Cr-Spl |
| Note: li = liquid. Mineral symbols after Kretz (1983). |
 Figure 7 A-B. Photomicrographs of Group IIHN xenoliths.
Figure 7 A-B. Photomicrographs of Group IIHN xenoliths.
A. Sample Hx14z. Large poikilitic Hbl surrounding anhedral, resobed Pl and Ol, suggesting a reaction relationship between the interstitial liquid, Pl and Ol to produce Hbl. Note the bent twins of the Pl crystal in the upper left corner of the picture (black arrow). B. Sample Hx14v. Poikilitic Phl surrounding anhedral Ol and subhedral Pl.
4. Mineral and glass compositions
Electron microprobe analyses (CAMECA SX-50, University of Lausanne) of olivine, clinopyroxene, orthopyroxene, hornblende, phlogopite, plagioclase, apatite, spinel, ilmenite, and glass were performed primarily as point analyses, but in some cases as core to rim traverses (not shown). Mineral names and structural formulae were determined following Morimoto et al. (1988) for pyroxenes, Leake et al. (1999) for amphiboles, Rieder et al. (1998), Deer et al. (1962) and Dymek (1962) for micas, Deer et al. (1992) for olivine, plagioclase, apatite, and Stormer (1983) for spinel and ilmenite. The operating conditions of the electron microprobe analyses are given in Appendix I.
4.1. Group I xenoliths. Partially crystallized olivine norites
4.1.1. Cumulus Cr-spinel, olivine and clinopyroxene
Cr-spinel has Cr2O3 contents varying from 10 to 18 wt %, Al2O3 from 5 to 17 wt %, and TiO2 from 0.2 to 8 wt % (Table 3). These compositions partly overlap with the field of spinel from volcanic rocks and ultramafic complexes, but commonly have higher (calculated) Fe2+ (Fig. 8). Olivine core to rim compositional traverses show constant to decreasing forsterite and NiO contents, with Fo86 and 0.46 wt % NiO in cores, and Fo76 and <0.05 wt % NiO in rims (Table 4). These high Fo and Ni contents indicate that the olivine crystallized from a basaltic magma.
Clinopyroxene is diopsidic, with a mg-number (mg-number= 100*Mg/(Mg+Fet) in mols; Fet= total iron) of 84 to 81, and Cr2O3 contents between 0.8 to 0.3 wt %. Their compositions expressed as quadrilateral end members, are Wo46-45, En46-44, Fs9-11 (Table 5). These compositions overlap with clinopyroxenes from water-bearing experiments of basalts and basaltic andesites (Fig. 9) which suggests that clinopyroxenes crystallized from a water-bearing basalt or basaltic andesitic magma (e.g., Gaetani et al., 1993).
4.1.2. Plagioclase
Plagioclase is compositionally zoned from 86 anorthite mol% (An86) in the cores to An20 in the rims (Table 6). There are two main textural types of plagioclase. Those that are not included in other minerals are characterized by bimodal compositions, with normally zoned cores of An86-78 and rims of An45-26. Cores and rims are separated by a compositional gap of ~ 35 mol% An; plagioclase with intermediate compositions (i.e., An60) is almost lacking (Fig. 10). Plagioclase included in hornblende, orthopyroxene, and phlogopite lacks the compositional gap and shows normal zoning profiles from An86 to An40, but with most compositions between An85 and An70 (Fig. 10). As the compositions of plagioclases included in hornblende, orthopyroxene, and phlogopite are the almost the same we infer that the three host minerals crystallized almost simultaneously. Assuming that the most anorthitic plagioclase core compositions (An86) crystallized from basaltic magmas similar to those erupted in the TSPC, the plagioclase crystallized from basaltic magmas with ~ 3 wt % H2O, (distribution coefficient of Sisson & Grove, 1993). For a detailed description of the plagioclase textures, major and trace element zoning see Chapter III.
 Figure 8. Composition of Cr-spinel and magnetites of the three groups of xenoliths.
Figure 8. Composition of Cr-spinel and magnetites of the three groups of xenoliths.
S & G (1993) are the spinel compositions (excepting two Cr-Al-Mg spinels of 79-35g and two magnetites not buffered at NNO) of the 2 kbar water-saturated crystallization experiments of Sisson & Grove (1993). Spinel compositions from volcanic rocks and those of Alaskan type ultramafic complexes taken from Beard & Borgia (1989), and from Conrad & Kay (1983). Figure modified from Sisson & Grove (1993).
4.1.3. Post-cumulus orthopyroxene, hornblende and phlogopite
Orthopyroxene is characterized by high mg-numbers (82-77) and high Cr2O3 contents (up to 0.4 wt %). Core to rim traverses show nearly constant mg-numbers whereas Cr2O3 concentrations vary irregularly.
Hornblende is magnesiohastingsite, with mg-numbers of 80-72 and Cr2O3 contents are commonly <0.30 wt %, but occasionally up to 1 wt % (Table 7). Core to rim compositional traverses show that TiO2 concentrations decrease (~ 4 wt % to ~1 wt %) whereas Al2O3, MgO, and Na2O increase slightly towards the rim. Some crystals are sector zoned and important compositional differences in Ti, Al, Mg and Ca occur across sectors. The Na/Ca values range from 0.3 to 0.7 (atoms per formula unit = apfu). These values are higher (for a given Al) compared to most hornblendes from other subduction-related gabbroic xenoliths and than those of most hornblendes crystallized from low pressure (< 3 kbar) experiments done on basaltic to andesitic compositions (see Fig.11).
Phlogopite has mg-numbers ranging form 82 to 77 and Cr2O3 contents are commonly <0.1, but up to 0.5 wt % (Table 8). Core to rim compositional traverses show that TiO2 concentrations slightly decrease, whereas MgO contents increase slightly towards the rim. The BaO and Cr2O3 concentrations are highly irregular. The Na2O contents are high (2 to 3.4 wt %), and Na/K values (apfu) range from 0.2 to 0.9. Such values are much higher than those of phlogopites from basaltic water-bearing low pressure experiments or the biotite phenocrysts in the host dacite lava (Fig. 12).
Noteworthy compositional features of orthopyroxene, hornblende, and phlogopite in these gabbroic xenoliths are their high mg-numbers and Cr2O3 contents (Figure 13). The Cr2O3 contents of these three minerals overlap with those of the clinopyroxenes, and are even higher in the case of hornblende. This is in accord with the interpretation that orthopyroxene, hornblende, and phlogopite are the products of reactions that involved an evolved water-rich liquid and early crystallized Mg and Cr-rich minerals (olivine, Cr-spinel, and clinopyroxene).
 Figure 9 A-C. Pyroxene compositions of the three xenolith groups.
Figure 9 A-C. Pyroxene compositions of the three xenolith groups.
For comparison are also shown those of the dacite lava and those of low pressure (<3 kbar) water saturated crystallization experiments of basalts and basaltic andesites (data of: Sisson & Grove, 1993; Gaetani et al., 1994; Grove et al., 1997; Moore & Carmichael, 1998). A. Cpx compositions of Group I xenoliths suggest that they crystallized at low pressure from water-bearing basaltic to basaltic andesitic magmas. B-C. Pyroxene composition of Group II are more difficult to interpret because they are exsolved.
4.1.4. Apatite
Apatites have F contents ranging from 0.3 to 0.8 wt % and Cl contents from 0.6 to 1.8 wt % (Table 9). Apatites from sample Hx14b have much lower mol Cl/F (0.4-0.6) than those of sample Hx14n (2.2 to 3.5). Calculated OH contents (OHc) are generally higher than those of apatites from tholeiitic layered intrusions (Fig. 14) probably reflecting the higher H2O contents of calc-alkaline versus tholeiitic magmas.
4.1.5. Glass
Glass from sample Hx14n ranges from dacitic to rhyolitic, with SiO2 contents varying from 66.7 to 71.6 wt %, Na2O from 2.6 to 6.6 wt %, and K2O from 3.7 to 8.6 wt %. Glass from sample Hx14b is rhyolitic, with SiO2 up to 75 wt % and lower Na2O (3.2 to 5.3 wt %) and K2O (3.6 to 5.5 wt %) than the glass of sample Hx14n (Table 10). The totals of the electron microprobe analyses are generally are between 97.6 and 100.7 wt % suggesting that the interstitial glass is largely but variably degassed.
 Figure 10 A-D. Histograms of plagioclase compositions of the three xenolith groups.
Figure 10 A-D. Histograms of plagioclase compositions of the three xenolith groups.
A. Group I. Pl not included in other minerals show a bimodal composition, with cores at An85-75 and rims at An45-30. Pl of intermediate composition (e.g., An60) are almost lacking. B. Group I. Pl included in Opx, Hbl and Phl have similar compositions (An85-35), suggesting that the three minerals co-crystallized. C. Most samples of Group IICL have Pl of An60-40 composition. Pl of samples Hx14y and Hx14e have higher An contents (~ An88-80). D. Group IIHN have mainly Pl with high An contents.
4.2. Group II. Xenoliths with subsolidus textures
4.2.1. Cr-spinel and olivine
Cr-spinels from Group IICL xenoliths have ~18 wt % Cr2O3, ~16 wt % Al2O3, and ~4 wt % MgO (Table 3). The (calculated) Fe2+ is higher than that of spinels from volcanic rocks (Fig. 8). Spinels from Group IIHN xenoliths have compositions that partly overlap with the compositional field defined by spinels produced in crystallization experiments of Sisson & Grove (1993) and with the field defined by volcanic spinels (Fig. 8 and Table 3).
Olivine of Group II xenoliths has lower Fo and NiO contents than those in Group I. Olivine of Group IICL is Fo81-72 with NiO contents <0.25 wt %, and that of Group IIHN is Fo79-78 with NiO concentrations <0.20 wt % (Table 4).
4.2.2. Pyroxenes
As pyroxenes are commonly exsolved, their compositions reflect the effect of subsolidus processes, precluding a straightforward estimate of their magmatic crystallization conditions. In Group IICL xenoliths, clinopyroxenes are diopsidic to augitic, with mg-numbers of 81 to 73 and Cr2O3 contents <0.1 wt % (Table 5). Their composition range from those of low pressure (< 3kbar) water-bearing crystallization experiments of basaltic to basaltic andesitic composition to more evolved than the clinopyroxene of the dacite host (Fig. 9). Orthopyroxene has mg-numbers of 80-65, and Cr2O3 contents <0.1 wt % (Table 5). Their compositions partly overlap with orthopyroxenes in Group I xenoliths but they extend to more evolved than the orthopyroxene of the host dacite (Fig. 9). In Group IIHN xenoliths, clinopyroxene is diopsidic to augitic with compositions that overlap with those of low pressure (< 3 kbar) crystallization experiments of basaltic to basaltic andesitic composition (Fig. 9). It has high mg-numbers (89 to 81) and relatively high Cr2O3 contents (up to 0.3 wt %). Orthopyroxene has mg-numbers of 81-77 and Cr2O3 contents <0.1 wt % (Table 5).
 Figure 11 A-C. Hornblende composition (atoms per formula unit) from the three groups of xenoliths.
Figure 11 A-C. Hornblende composition (atoms per formula unit) from the three groups of xenoliths.
For comparison those of the dacite lava, those of low pressure (< 3 kbar) water-bearing crystallization experiments of basaltic to andesitic composition and those of gabbroic xenoliths from subduction-related volcanoes are also shown. A. Group I xenoliths. B. Group IICL xenoliths. C. Group IIHN xenoliths. Data sources: experiments (Rutherford & Devine, 1988; Sisson & Grove, 1993; Grove et al., 1997; Moore & Carmichael, 1998; BC in prep. is Barclay & Carmichael). Xenoliths: Aleutians (Conrad & Kay, 1983 and Debari et al., 1987), Lesser Antilles (Arculus & Wills, 1980), Medicine Lake (Grove & Donnelly-Nolan, 1986), Arenal (Beard & Borgia, 1989), Japan (Aoki, 1971), Mt. Pelee (Fichaut et al., 1989), and Mt. St. Helens (Heliker, 1995).
4.2.3. Plagioclase
In Group IICL xenoliths, plagioclase ranges from An88 to An45 (Table 6). The histogram of plagioclase compositions shows two modes, one at An85-80 and another at An60-55 (Fig. 10). The two modes reflect the fact that some samples have mainly anorthitic plagioclase (e.g., Hx14e, Hx14y), whereas in others plagioclase is more albitic (e.g., Hx14a). In general, plagioclases are normally zoned.
Plagioclase of Group IIHN xenoliths ranges from An88 to An50, but most plagioclases are between An88 and An80 (Fig. 10). Core to rim compositional profiles show constant or decreasing anorthite towards the rim.
4.2.4. Hornblende and phlogopite
Hornblende of Group IICL xenoliths is commonly magnesiohastingite, but some tschermakitic hornblendes and magnesiohornblendes are also present in sample Hx14y (Table 7). They have mg-numbers ranging from 77 to 64, and Cr2O3 contents <0.05 to 0.55 wt %. The Na/Ca values (0.2-0.5) of most hornblendes overlap with those of subduction-related gabbroic xenoliths and with those of low pressure (< 3 kbar) experiments done on basaltic to andesitic compositions (Fig. 11). Hornblende of Group IIHN xenoliths is magnesiohastingite (Table 7), with mg-numbers ranging from 80 to 72, and Cr2O3 contents are commonly <0.1 wt % (occasionally up to 0.5 wt %). The Na/Ca values vary between 0.3 and 0.5. These values are higher than those of Group IICL xenoliths and higher than most hornblendes from other xenoliths and experiments, but lower than hornblendes of Group I xenoliths (Fig. 11).
Phlogopite of Group IILC xenoliths has mg-numbers of 81-70 and Cr2O3 contents between <0.15 and 0.3 wt % (Table 8). These phlogopites also have high Na2O contents (1-2 wt %), although lower than in phlogopites of Group I xenoliths. Their Na/K values (0.18-0.39) partly overlap with those of low pressure water-bearing crystallization experiments (Fig. 12). The compositions of late phlogopites are within the compositional ranges of poikilitic phlogopite.
Phlogopite of Group IIHN xenoliths has mg-numbers of 84-77, and Cr2O3 contents up to 0.2 wt %, but typically is between 0.1-0.05 wt % (Table 8). The phlogopites are also rich in Na2O, with most crystals having between 1.5 at 2.5 wt %, but one crystal with up to 5 wt %. Their Na/K values are very high, ranging from 0.2 to 2.2. These values are higher than those of phlogopite from the other groups of xenoliths or those of phlogopite from low pressure experiments (Fig. 12). For a more detailed discussion of these Na-rich phlogopites see Chapter IV.
 Table 8. Representative compositions of phlogopites
Table 8. Representative compositions of phlogopites
The Cr2O3 contents of hornblende and phlogopite from Group II xenoliths range from concentrations that overlap with those of clinopyroxene to higher concentrations (Fig. 13). Because hornblende and phlogopite crystallized late and they occur as mantles around resorbed pyroxenes and olivine (with Cr-spinel inclusions), we interpret these high Cr2O3 contents as indicative of reactions between Cr-bearing minerals and water-rich evolved liquid.
4.2.5. Magnetite and Ilmenite
Poikilitic magnetite and ilmenite are commonly exsolved. Magnetite varies from Ulv0.04 to Ulv0.44 and ilmenite from Ilm0.74 to Ilm0.96.
4.2.6. Apatite
Apatites of Group IICL xenoliths have Cl contents ranging from 1.3 to 2.9 wt % and F concentrations from 0.2 to 0.9 wt % (sample Hx12a up to 1.7 wt %). The Cl/F values (apfu) range from 0.5 to 2.7 (Table 9). Apatites of Group IIHN xenoliths have concentrations of F (0.2 to 0.7 wt %) and Cl (1.8 to 2.8 wt %) within the range of the apatites in the other groups of xenoliths. Most apatites have Cl/F values of ~2, except one analysis (8). The halogen contents of the apatites in Group II xenoliths vary from similar to those of layered intrusions to higher OHc abundances (Fig. 14).
 Figure 12 A-C. Phlogopite composition (atoms per formula unit) from the three groups of xenoliths.
Figure 12 A-C. Phlogopite composition (atoms per formula unit) from the three groups of xenoliths.
For comparison are also shown those of the dacite lava, and those of low pressure (< 3 kbar) water-bearing crystallization experiments of basaltic to andesitic composition. A. Group I xenoliths. B. Group IICL xenoliths. C. Group IIHN xenoliths. Data sources: SG, 1993 is Sisson & Grove, 1993; RC, 1996 is Richter & Carmichael, 1996; and BC, in prep. is Barclay & Carmichael.
4.2.7. Glass
The interstitial glass in two samples (Hx12a and Hx14w) of Group IICL xenoliths is rhyolitic, with SiO2 contents ranging from 71 to 74 wt %, Al2O3 from 11.8 to 14 wt %, Na2O from 2 to 3.4 wt %, and K2O from 5.7 to 6.7 wt % (Table 10).
4.2.8. Minerals filling microfractures
The compositions of the minerals that are present in microfractures of Group IICL xenoliths typically overlap with those that are poikilitic or in the matrix. Orthopyroxene has mg-numbers of 78-73, Cr2O3 contents are <0.1 wt %, overlapping in composition with those found in the matrix (Fig. 9). Hornblende has mg-numbers of 77-66, Cr2O3 contents <0.1 wt %. In general, it has a low Na/Ca (0.2-0.4) when compared to poikilitic hornblendes of Groups I and IIHN xenoliths (Fig. 11).
Phlogopite has slightly lower mg-numbers (76-70), lower contents of Cr2O3 (<0.15 wt %), Na2O (0.7-1.3 wt %) and lower Na/K values (0.1-0.2) than those of poikilitic phlogopite (Fig.12). The Fe-Ti oxides have highly variable compositions. Ilmenite varies from Ilm0.54 to Ilm0.82, and magnetite from Ulv0.05 to Ulv0.55 (Table 3).
 Figure 13 A-C. Concentrations of Cr2O3 wt % of mafic minerals from the three groups of xenoliths.
Figure 13 A-C. Concentrations of Cr2O3 wt % of mafic minerals from the three groups of xenoliths.
A. Group I. Note the high Cr2O3 contents of Hbl (up to 1.2 wt %), Phl (up to 0.8 wt %) and Opx (up to 0.5 wt %) that may be higher than in the Cpx (up to 0.9 wt %). B-C. Hbl and Phl from Group II xenoliths also have high Cr2O3 concentrations (up to 0.4 and 0.6 wt % respectively) but lower than those of Group I xenoliths. See text for discussion.
 Figure 14. Apatite halogen composition of the three xenolith groups.
Figure redrawn from Boudreau (1995). OHc indicates calculated OH from structural formula.
Figure 14. Apatite halogen composition of the three xenolith groups.
Figure redrawn from Boudreau (1995). OHc indicates calculated OH from structural formula.
The field of layered intrusions includes analyses from Skaergaard, Jimberlana, Dufek, Munni Munni, Penikat, Great Dyke, Mt. Thirsty, Ora Banda Sill, Windimurra, Stillwater and Bushveld (not below major PGE-bearing zones). Apatites of the three groups of xenoliths tend to have higher OHc than Ap of layered intrusions, which may reflect the higher water contents of calc-alkaline gabbros with respect to tholeiitic intrusions. The Ap with the lowest Cl/F is of sample Hx12a. References can be found in Boudreau (1995).
5. Whole-rock chemical composition
The major, minor and trace element compositions of the xenoliths were determined by X-Ray Fluorescence (XRF) at the University of Massachusetts, whereas the rare earth elements (REE) were analyzed by Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AES) at the University of Geneva (Table 11). To assess whether the bulk chemistry of the xenoliths has been affected by mineral accumulation or open-system percolative processes (e.g., melt migration), we will compare the compositions of the xenoliths to the mean composition of ten basalts of the TSPC assumed to be representative of liquids. The major, minor and trace element composition of the basalts was also determined by XRF at the University of Massachusetts. The REE were determined by Instrumental Neutron Activation Analysis (INAA) at the University of Massachusetts. Two samples were analyzed for their REE concentrations by ICP-AES and INAA and they agree within 10%. Analytical methods and precision of the analyses are given in Appendix I.
5.1. Group I. Partially crystallized xenoliths
The major element compositions of these xenoliths are characterized by low SiO2 (46-47 wt %) and high MgO (20-21 wt %) when compared to the basaltic composition of the TSPC (Table 11 and Fig. 15). The xenoliths have low concentrations of incompatible minor and trace elements (K2O = 0.52-0.71 wt %; Rb =10-12 ppm; Zr = 53-56 ppm; Y = 8-9 ppm), whereas concentrations of Ni (446-643 ppm), and Cr (707-1308 ppm) are much higher than those of the TSPC basalts (Table 11). The high MgO, Ni, and Cr concentrations together with the high modal proportions of olivine suggest that the xenoliths have accumulated olivine (and Cr-spinel) (Fig. 15). Ratios of elements that are not affected by olivine accumulation, such as Ca/Na mol (2.4-3.1), K/P (8-13), P/Zr (9-10), and Rb/Y (1-1.5) fall within the range of TSPC basalts (Fig. 16). The REE abundances are low (e.g., Ce = 12.4-15.5 ppm), and the La/Yb values are within the mean of the TSPC basalts (La/Yb = 8.1-10.6) (Fig. 17).
5.2. Group IICL xenoliths
The major element compositions of most of these xenoliths are comparable to those of high-alumina basalts typical of subduction zones (e.g., Gust & Perfit, 1987; Kersting & Arculus, 1994). The SiO2 concentrations range from 49 to 52 wt %, MgO from 5.2 to 8.2 wt %, Al2O3 from 17.2 to 19 wt %, and Ca/Na mol from 2.3 to 3.8 (Table 11). Samples Hx14e, Hx14h, and Hx14y stand out from the rest by their lower concentrations of SiO2 (46-47 wt %), generally higher MgO (7.6-10.7 wt %), higher Al2O3 (21.8-23 wt %), and higher Ca/Na (4.4-7.7) (Fig. 15). Trace and minor element abundances are highly variable. Most xenoliths have concentrations of incompatible elements that range from those like TSPC basalts to much lower values (Fig. 15). For example, concentrations of K2O range from 0.2 to 0.8 wt %, Rb from 3 to 40 ppm, Zr from 7 to 84 ppm, and Y from 5.3 to 23.4 ppm (Table 11). The P2O5 and TiO2 also show large concentration ranges, from lower to higher values than those of the TSPC basalts. In contrast, the Sr and Ni concentrations of most xenoliths fall within the concentration ranges of the TSPC basalts (Fig.15). Samples Hx14e, Hx14h, and Hx14y also have lower concentrations of some incompatible elements (i.e., Y and Zr), but the Ni concentrations (133-237 ppm) are higher than most other xenoliths, whereas K2O (0.34-2.48 wt %) and Rb (10-80 ppm) are highly variable. The low concentrations of incompatible element of most xenoliths could be due to accumulation of plagioclase or olivine. However, the fact that most xenoliths have Sr and Ni concentrations within the values of the TSPC basalts suggests that loss of interstitial melt rich in incompatible elements is a more plausible explanation. Accumulation of olivine may be a contributing factor for samples Hx14e, Hx14h, and Hx14y, as they have higher Ni concentrations than the rest of the xenoliths and the TSPC basalts.
Ratios of incompatible elements are also highly variable. Most of the xenoliths have values of K/P (5-76) that range from higher to lower than those of the TSPC basalts. The P/Zr (11-38) and Rb/Y (0.4-27) values vary from those of the TSPC basalts to higher (Fig.16). Samples Hx14e, Hx14h, and Hx14y have P/Zr values (10-11) that fall within those of the TSPC basalts, whereas Rb/Y (2-27) and K/P (8-78) range from values of the TSPC basalts to much higher (Fig. 16). Such a large range of incompatible element ratios further indicates that open-system processes (e.g., melt or fluid migration) may have modified the bulk chemistry of the xenoliths.
The REE contents are generally low, but range from higher (e.g., Ce = 34.8 ppm) and steeper patterns (La/Yb = 11.9) than those of the TSPC basalts, to lower REE concentrations (e.g., Ce = 7 ppm) and less steeper patterns. Some xenoliths show a positive Eu anomaly (Eu/Eu* up to 2.5) when normalized to primitive mantle (McDonough et al., 1992). In general, samples with lower REE concentrations have higher positive Eu anomalies, and their REE patterns are flatter (Fig. 17). Positive Eu anomalies can be produced by plagioclase accumulation, although they could also occur if the parent liquid had initially a positive Eu anomaly or, as it will be discussed later, by loss of interstitial liquid with a negative anomaly. The REE concentrations of samples Hx14e, Hx14h, and Hx14y are low (e.g., Ce = 10-11 ppm) with the HREE (Heavy Rare Earth Elements, Gd to Lu) below determination limits (Fig. 17).
5.3. Group IIHR xenoliths
These samples have low concentrations of SiO2 (~ 45 wt %) and high MgO (13.3-16.5 wt %) when compared to the mean basaltic composition of the TSPC (Fig. 15). The concentrations of incompatible elements are also low (K2O = 0.26-0.47 wt %, Zr = 28-30 ppm, and Y = 3.5-4.2 ppm). The concentration of Ni (226-335 ppm) and the Ca/Na values (5.2-6.3) are higher than those of the TSPC basalts, thus olivine and plagioclase accumulation could be partly responsible for the low incompatible element abundances. Ratios of incompatible elements such as P/Zr (6-10) are within the values of the TSPC basalts, but the K/P (7-25) and Rb/Y (2-3) values range from those of the TSPC basalts to higher (Fig. 16). This indicates that processes other than plagioclase and olivine accumulation have modified the composition of these samples (e.g., melt or fluid migration; see Discussion). They have very low REE concentrations (e.g. Ce= 6.7-7.9 ppm), with the HREE below detection limits (Fig. 17).
 Figure 15. Major and trace element variation diagrams of the three groups of xenoliths.
Figure 15. Major and trace element variation diagrams of the three groups of xenoliths.
Also shown is the mean composition of ten TSPC basalts. Black arrows indicate the effects of accumulation of Pl and Ol to the TSPC composition. Ni concentration is that of Ol analysis n-V-1c. The Sr concentration of An88 and An60 is taken from the isotope dilution analyses of Pl of samples Hx14v and Hx14a, respectively (Table 12). See text for discussion.
 Figure 16. Variation diagrams of element ratios of the three groups of xenoliths.
Figure 16. Variation diagrams of element ratios of the three groups of xenoliths.
The legend is the same as in Fig. 15. The arrow with the "a" letter indicates the geochemical effect (qualitative) of removing interstitial melt (after apatite crystallization) to a bulk composition of the TSPC. The arrow with the "b" letter indicates the geochemical effect (qualitative) of addition of a fluid phase. See text for discussion.
 Figure 17 A-C. Rare earth element concentrations normalized to primitive mantle (McDonough, 1992).
Figure 17 A-C. Rare earth element concentrations normalized to primitive mantle (McDonough, 1992).
A. Group I xenoliths. B. Group IICL xenoliths. C. Group IIHN xenoliths. See text for discussion.
6. 40Ar/39Ar analyses and Sr isotope composition
6.1.40Ar/39Ar analyses
Incremental furnace heating 40Ar/39Ar analyses were performed at the University of Geneva following the methods described in Singer & Pringle (1996). Hornblende separates from the two samples of Group IIHN xenoliths show comparable apparent age spectra (Fig. 18), but they do not define a plateau that could be used to derive a reliable age (e.g., Singer & Pringle, 1996). Apparent ages increase with temperature from ca. 0.5-3 Ma (at 750 oC) to 8-9 Ma (at 1050 oC), and then decrease to ca. 4 Ma (at 1200 oC).
The high K/Ca of the first temperature steps (up to 950 oC) suggest the presence of phlogopite in the mineral separate, but afterwards the K/Ca value remains low (0.04-0.05) and within the values of hornblende obtained by electron microprobe analyses (0.03-0.08). This suggests that hornblende was the main degassing mineral (Fig. 18). Determining the cause(s) of the discordant 39Ar release spectra is beyond the scope of this paper, but since the samples are xenoliths, heat from the host lava may have modified their Ar distribution and partially degassed the hornblendes. In any case, we interpret these Ar data as indicating that the xenoliths are certainly older than 1 Ma and may be up to 8 Ma. The total fusion age is ca. 5 Ma, which is ~1 Ma younger than the age of the Huemul and Cerro Risco Bayo plutons (6-6.4 Ma; Nelson et al., 1999) that form the basement of the TSPC.
 Figure 18. Results of 40Ar/39Ar analyses of two hornblende separates (samples Hx14v and Hx14z) of Group IIHN xenoliths. See text for discussion.
Figure 18. Results of 40Ar/39Ar analyses of two hornblende separates (samples Hx14v and Hx14z) of Group IIHN xenoliths. See text for discussion.
6.2.87Sr/86Sr and Rb-Sr concentrations.
87Sr/86Sr plus Rb and Sr concentrations of bulk-rock, hornblende and plagioclase mineral separates were determined by Thermal Ionization Mass Spectrometry at the University of California at Los Angeles (Table 12). Two samples of Group I xenoliths have indistinguishable 87Sr/86Sr of 0.703948-0.703955, which are lower than those of Group II xenoliths and among the lowest of the entire volcanic complex (J. Davidson and M. Dungan, unpublished data).
As the 40Ar/39Ar analyses suggest that Group II xenoliths are < 9 Ma and they have low Rb concentrations, the reported Sr isotopic compositions are close to the initial values. Whole-rock and plagioclase 87Sr/86Sr of three samples of Group IICL xenoliths vary from 0.704009 to 0.704117, which are within the values of the majority of the lavas erupted in the TSPC. The bulk-rock, plagioclase, and hornblende 87Sr/86Sr of sample Hx14v from Group IIHN xenoliths are within error (0.704048-0.704060). Those of sample Hx14z are also within error (0.704037-0.704021) but slightly lower than those of sample Hx14v. The small range of 87Sr/86Sr (< 0.00014) of minerals and bulk-rock of all xenoliths suggests that their composition has not been modified by interaction with radiogenic liquids.
7. Discussion
7.1. Interpretation of mineral and whole-rock compositions of Group I xenoliths
7.1.1. Crystallization sequence
We propose a differentiation history for these xenoliths consisting of two stages: (1) crystallization and accumulation of olivine, Cr-spinel, and clinopyroxene from a basaltic water-bearing magma, and (2) reaction of an evolved liquid with the cumulus minerals to produce orthopyroxene, hornblende, and phlogopite with high mg-numbers and Cr2O3 contents (Fig.13). Co-crystallization of hornblende and orthopyroxene (and to some extent phlogopite) is indicated by the fact that they include plagioclase with the same composition (Fig. 10). Plagioclases are not resorbed suggesting that they did not participate in the reactions. Given the bimodal distribution of plagioclase composition, we assign the crystallization of anorthitic plagioclase cores (An85-70) to the first crystallization stage, and the more albitic plagioclase rims (An45-20) to the second stage.
The first crystallization event agrees well with crystallization sequences reported from low pressure (< 3 kbar) experiments on water-bearing basaltic and basaltic andesitic magmas (e.g., Sisson & Grove, 1993; Moore & Carmichael, 1998; Barclay & Carmichael, in prep.). The second stage is more unusual. Reactions between the interstitial liquid and early crystallized mafic minerals could be, in principle, due to: (1) closed-system crystallization of a water-bearing basaltic magma or (2) open-system percolative processes involving displacement of the mafic interstitial liquid by ingress of a more differentiated melt (e.g., infiltration metasomatism; Irvine, 1980). We favor the second possibility for the following reasons:
(1) Reaction relations between clinopyroxene or olivine and liquid to produce hornblende, and between olivine and liquid to produce orthopyroxene have been reported in low pressure (< 3 kbar) crystallization experiments of basaltic to andesitic composition (e.g., Sisson & Grove, 1993; Grove et al., 1997; Moore & Carmichael, 1998; Barclay & Carmichael, in prep.). However, in none of these experiments co-crystallization of hornblende and orthopyroxene (and phlogopite) in a reaction relation with olivine or clinopyroxene has been reported. Phlogopite is not reported even in water-bearing experiments with crystallinities of up to 90 % (Kawamoto, 1996).
(2) The Na/Ca values of hornblende are high (for a similar Al content) with respect to hornblendes in most subduction-related gabbroic xenoliths and with respect to hornblende in low pressure (< 3 kbar) experiments on mafic to intermediate magmas (Fig. 11). This could be due to the high Na/Ca of the inferred reacting liquid (e.g., dacites of Volcán San Pedro have Na/Ca of ~ 1.2).
(3) Primary magmatic micas in gabbroic xenoliths or plutons are rare, and when they occur they are biotite with much lower Mg/Fet than the phlogopite reported here (e.g., Arculus & Wills, 1980). Although phlogopite and biotite are not commonly analyzed in studies of gabbroic rocks, the Na/K of most phlogopites present in the xenoliths is higher than any of the phlogopite compositions reported from low pressure (< 3kbar) crystallization experiments (Fig. 12). The presence of phlogopite suggests that the reacting liquid had high concentrations of K2O and H2O, whereas the high Na/K could be due to the high mg-number of the phlogopite, as Volfinger et al. (1985) suggested that micas with high Mg/Fet preferentially incorporate Na into its structure
(4) The abrupt compositional change in plagioclase from An85-70 to An45-20 between cores and rims is difficult to explain in terms of closed-system crystallization (e.g., Brophy et al., 1996). Plagioclase consisting of an anorthite-rich core mantled by an albite-rich rim is common in cases of mingling between felsic and mafic magmas (e.g., Feeley & Dungan, 1996), and suggests a two-stage history for the plagioclases of these gabbroic xenoliths.
(5) The coexistence of forsteritic olivine and rhyolitic glass is atypical of closed-system crystallization, but examples of such occurrence have been recognized in systems where mingling between felsic and mafic magmas has occurred (e.g., Feeley & Dungan, 1996)
To reconcile all these observations we envision a scenario where the xenoliths consisted of a crystal network of olivine, Cr-spinel, clinopyroxene and anorthitic plagioclase with mafic liquid filling the interstices. The mafic liquid was displaced through porous flow by a more evolved melt that reacted with the cumulus minerals and triggered crystallization-reaction of hornblende, orthopyroxene, and phlogopite. This was followed by crystallization of albitic plagioclase rims.
The process of melt migration was probably driven by the density contrast between the intercumulus mafic melt and the lighter and more evolved liquid that displaced it. Such a process has been reproduced in laboratory crystallization experiments using solutions of simple salts (e.g., Chen & Turner, 1980; Kerr & Tait, 1986; Tait & Jaupart, 1992). In these experiments, light residual liquid released from the lower parts of the crystal-rich solution migrates upward and causes dissolution of the overlying crystals (Tait & Jaupart, 1992). We propose that the xenoliths represent the natural counterparts of such a system wherein light residual liquid dissolved and reacted with cumulus minerals. In the following sections we attempt to derive some constraints on the composition and amount of the percolating liquid.
7.1.2. Constraints on the amount of the reactive melt
The proportions of post-cumulus minerals (hornblende, orthopyroxene, phlogopite, plus plagioclase rims) and glass present in the xenoliths provide a rough estimate of the amount of interstitial melt that could have been displaced. However, since hornblende, orthopyroxene, and phlogopite are the products of reactions that consumed liquid and minerals, their observed modal abundances do not directly correspond to the porosity at the time of melt migration. The proportions of liquid and minerals that participated in the reactions were taken from the literature: for the hornblende reaction we have used the stoichiometry (wt %) determined by Sisson & Grove (1993): 100 Hbl = 22 Ol + 38 Cpx + 42 liquid. For the orthopyroxene reaction we have estimated the stoichiometry (wt %) suggested by Kelemen (1990): 100 Opx = 60 Ol + 40 liquid. No stoichiometry for the phlogopite reaction was found in the literature, so we assumed that the modal amount of phlogopite corresponds to liquid consumed. Lastly, we have estimated that plagioclase rims are 1/3 of the plagioclase in the xenoliths.
The results are shown in Table 13. It is apparent from the calculations that: (1) prior to reaction the three xenoliths consisted of large amounts of olivine (40-43 wt %), so that for sample Hx14n the calculated amount of olivine prior to reaction is twice the observed amount. The amount of clinopyroxene that was consumed (4.5-12.5 wt %) is also significant, (2) The quantity of reactive liquid is almost the same for the three xenoliths (31 to 33 wt %). This implies that prior to melt migration, the porosity of the xenoliths was ~ 0.3. The calculated amounts of crystals and melt are used in the following section to place some constraints on the composition of the reactive melt.
7.1.3. Constraints on the composition of the reactive melt
To further constrain the hypothesis that an evolved melt displaced the interstitial liquid of the xenoliths we have performed a mixing model between the modal composition of the cumulates prior to reaction (calculated previously) and a dacite from Volcán San Pedro. To account for the fact that in the previous calculations it was not possible to estimate the amount of Cr-spinel that was consumed in the hornblende and orthopyroxene reactions, we have added ~ 3 wt % of Cr-spinel to the composition of the cumulate. The results (Table 14) show that mixing 70 wt % of cumulate with 30 wt % of dacite produces compositions that resemble those of the bulk composition of the xenoliths, with residuals between 1.4 and 3.4. Given the uncertainties on the mineral modes estimated by the stoichiometry of the reactions taken from the literature, the low residuals seem to favor the hypothesis that the interstitial melt of the xenoliths was displaced by a dacitic liquid.
7.2. Interpretation of mineral and whole-rock compositions of Group IICL xenoliths
7.2.1. Crystallization sequence
The crystallization history of most of these xenoliths (excepting samples Hx14e, Hx14h and Hx14y) consisted of plagioclase±olivine, and later, pyroxenes and minor Fe-Ti oxides. This was followed by plagioclase, apatite, and by poikilitic magnetite and ilmenite. Hornblende and phlogopite post-date all other minerals and crystallized in a reaction relation with plagioclase, pyroxenes, and olivine.
The occurrence of late poikilitic Fe-Ti oxides, the low anorthite content of plagioclase (e.g., An60), and the relatively low enstatite contents of the orthopyroxene (e.g., En62), suggest a drier crystallization sequence than Group I xenoliths. Samples Hx14e, Hx14h, and Hx14y lack the late poikilitic Fe-Ti oxides, and have plagioclases with high anorthite contents (e.g., An88), suggesting they crystallized from wetter magmas than the rest of the xenoliths of this group.
7.2.2. Geochemical evidence for melt or fluid migration
As previously discussed, the low concentrations of incompatible elements (i.e., Zr) of most xenoliths are not correlated with high concentrations of compatible elements (i.e., Sr and Ni). This suggests that the low incompatible element concentrations are due to loss of evolved intercumulus liquid rather than to mineral accumulation. As illustrated in the Ca/Na versus Sr plot, sample Hx14w is the only that lies on a mixing line between the composition of the TSPC basalts and plagioclase (An60), indicating that is the only xenolith that has accumulated a substantial amount of plagioclase. Samples Hx14e, Hx14h, and Hx14y have high Ni concentrations and high Ca/Na relative to the TSPC basalts so their low incompatible element abundances could be partly explained by accumulation of olivine and plagioclase (Fig. 16).
Loss of interstitial melt from a crystal pile consisting of plagioclase and pyroxenes should not greatly modify ratios of incompatible elements such as P/Zr and K/P, as the partition coefficients for these elements in plagioclase and pyroxenes are < 0.1 (e.g., Rollinson, 1993). To explain the large range of P/Zr displayed by the xenoliths we suggest that expulsion of intercumulus liquid occurred both prior to and after apatite crystallization. Samples with high P/Zr lost melt after apatite crystallization, whereas xenoliths with P/Zr values within the range of the TSPC basalts (but low Zr and P2O5 concentrations) lost melt prior to apatite crystallization (Fig. 16). It is worth pointing out that the xenoliths that lost melt prior to apatite crystallization are commonly the same that show positive Eu anomalies. This suggests that the positive Eu anomalies could be due to loss of interstitial liquid rich in REE with a negative Eu anomaly.
Ratios of incompatible elements involving Rb and K are also highly variable. For example, many samples have Rb/Y values similar or lower than those of the TSPC basalts, whereas others have very high Rb/Y. Since Y is highly compatible in apatite (e.g., Pearce & Norry, 1979), the low Rb/Y values of some xenoliths could be explained by loss of interstitial liquid after apatite crystallization. The high Rb/Y of other xenoliths suggest that they have gained Rb with respect to Y. The same arguments are valid if K is substituted for Rb, and if Y is substituted by Zr. Decoupling of K and Rb from the rest of incompatible elements can be produced by the involvement of an aqueous fluid phase. This is because the fluid-melt partition coefficients of K and Rb are much higher than those of Y and Zr (e.g., Keppler, 1996). Accordingly, the high Rb and K2O concentrations and the high Rb/Y (or low P/Rb) values of some xenoliths could be explained by the arrival of an aqueous fluid phase (in the form of bubbles) that dissolved into the remaining melt (= fluid fluxing). Fluid could have flowed along grain boundaries of crystals or through microfractures (e.g., Shinohara & Kazahaya, 1995).
The processes of melt and fluid migration discussed up to now are illustrated in qualitative terms in the P/Zr versus P/Rb plot of Figure 16. With respect to the values of the TSPC basalts, some xenoliths show P/Zr values that increase with P/Rb reflecting loss of melt after apatite crystallization (indicated by an arrow labeled a). The geochemical signature of fluid fluxing is to decrease the P/Rb values (indicated by an arrow labeled b) but maintain constant P/Zr, a trend that could be inferred for some samples.
7.2.3. Textural and mineralogical evidence for melt or fluid
The bent laths and microcracks displayed by the plagioclases and the microfractures filled with hornblende, phlogopite, orthopyroxene, and magnetite are interpreted as textural evidence of expulsion of interstitial liquid by compaction processes. Microfractures commonly cut across bent plagioclase crystals, suggesting that rock deformation changed from ductile to brittle (e.g., Kronenberg & Shelton, 1980), or that the behavior of plagioclase changed from plastic to cataclastic (e.g., Hacker & Christie, 1990). Perhaps, during initial ductile deformation of the crystal matrix, melt migrated through the pore spaces, and later during brittle deformation melt migration occurred mostly through microfractures. Deformed plagioclase crystals and veins filled with hydrous minerals have been also recognized in tholeiitic layered intrusions and interpreted as evidence of compaction and melt migration (e.g., Skaergaard, McBirney & Hunter, 1995; McBirney & Nicolas, 1997; Stillwater Complex, Meurer & Boudreau, 1998).
Because microfractures are mainly filled with hornblende and phlogopite, the percolating liquid was evolved and water-rich. This is in accord with the high P/Zr of many xenoliths which is consistent with melt expulsion at an advanced stage of evolution, after apatite crystallization. The inferred upward migrating liquid reacted with olivine, pyroxenes, and plagioclase, and produced hornblende and phlogopite with higher mg-numbers and Cr2O3 contents than those occurring in microfractures.
The evidence of fluid fluxing inferred from the high Rb/Y and low P/Rb values of some xenoliths can be documented by monitoring the halogen compositions of apatite (e.g., Boudreau & McCallum, 1989). When a fluid is exsolved from a melt, Cl tends to partition into the fluid phase, whereas F remains in the melt (e.g., Candela & Piccoli, 1995; Villemant & Boudon, 1999). Apatites crystallized from melts fluxed by fluids tend to have higher Cl/F values than those that crystallize from non-fluxed melts (e.g., Boudreau & McCallum, 1989). Apatites from one sample (Hx12a) have low Cl/F values (Fig. 14), whereas the rest have much higher Cl/F suggesting that they have crystallized from melts enriched by fluids. Because it is not apparent from Figures 14 and 16, it is worth noting that samples which lost melt prior to apatite crystallization, also have apatites with high Cl/F (e.g., Hx14y and Hx14e). This suggests that fluid arrival might have post-dated melt migration. Other mineralogical observations that are consistent with late-stage arrival of a fluid are the occurrence of radial aggregates of late phlogopite surrounding olivine, and in some cases orthopyroxene.
The available stable isotope analyses (sample Hx14h, whole rock: d18O = 5.4, plagioclase, d18O = 6.2; sample Hx14a, plagioclase, d18O = 6.3, all values relative to SMOW; B.S. Singer, unpublished data) suggest that the xenoliths have not been affected by hydrothermal meteoric water circulation (e.g., Taylor and Forester, 1979), so that the processes described here involved high temperature magmatic fluids.
7.3. Interpretation of mineral and whole-rock compositions of Group IIHN xenoliths
7.3.1. Crystallization sequence
A detailed crystallization sequence for this group of xenoliths is difficult to establish due to the high proportions of hornblende. However, at least two crystallization events can be distinguished: (1) crystallization of Cr-spinel, olivine, plagioclase, clinopyroxene, and orthopyroxene, and (2) reaction of an evolved water-rich liquid with the pre-existing minerals to produce poikilitic hornblende and phlogopite. The high anorthite contents of plagioclase (An88) suggest that the original magma was more water-rich than most xenoliths of Group IICL.
7.3.2. Geochemical evidence for melt and fluid migration
The bulk-rock compositions of these xenoliths are characterized by low concentrations of incompatible elements and high abundances of Ni and high Ca/Na, when compared to the TSPC basalts (Figs. 15 and 16). As hornblende crystallization apparently involved reactions that consumed olivine and plagioclase, it is difficult to establish from the observed modal abundaces whether the low incompatible element concentrations of these xenoliths are due solely to plagioclase and olivine accumulation, or if melt migration was also important. Ratios of incompatible elements such as P/Zr and P/Y values are within those of the TSPC basalts, which suggests that if migration of interstitial liquid occurred, it was prior to apatite crystallization. In contrast, their Rb/Y and K/P values are typically higher than those of the TSPC basalts. Using the same arguments presented for the Group IICL xenoliths, we suggest that a fluid fluxed the interstitial liquid of these xenoliths.
7.3.3. Textural and mineralogical evidence for melt and fluid migration
Because plagioclase inside hornblende shows bent twins (Fig. 7) it seems that hornblende (and probably phlogopite) crystallized after some deformation had already occurred. This is consistent with the interpretation that compaction of the cumulate pile and expulsion of interstitial melt is partly responsible for the low incompatible element concentrations of these xenoliths. We propose that after some interstitial melt escaped from the crystal-mush, a fluid enriched the residual liquid in volatile components including K, Rb, and H2O. This triggered reactions between the cumulus minerals and the liquid and produced hornblende and phlogopite with high mg-numbers and Cr2O3 contents. The process proposed here might be analogous to the situation described by Boudreau (1999) for the Olivine-Bearing Zone I of the Stillwater Complex (Montana). He interpreted the occurrence of hornblende and biotite mantling resorbed plagioclase and olivine as due to fluid fluxing and reactions.
Additional evidence for arrival of an aqueous fluid are the halogen contents of apatite (Fig. 14). Apatites from this group of xenoliths have high Cl/F values, and overlap with the samples of Group IICL for which fluid fluxing was proposed. The few available stable isotope analyses of this group of xenoliths (sample Hx14v: hornblende, d18O = 5.3; plagioclase, d18O = 6.1; bulk-rock, dD = -62, all values relative to SMOW; B.S. Singer, unpublished data) suggest that the fluids that fluxed the xenoliths were magmatic and not meteoric (e.g., Taylor & Forester, 1979).
7.4. Implication for the presence of hornblende and phlogopite in calc-alkaline gabbroic rocks
The three groups of gabbroic xenoliths have significants amounts of hornblende and phlogopite, either as small crystals filling microfractures, or as large poikilitic post-cumulus crystals that can make up >50 vol. % of the rock. In this respect they are not unusual, and a survey of the literature shows that the majority of calc-alkaline gabbroic xenoliths and plutons have important amounts of hornblende (xenoliths: Aoki, 1971, Itinome-gata, Japan; Arculus & Wills, 1980, Lesser Antilles; Conrad & Kay, 1984, Adak, Aleutian arc; Grove & Donnelly-Nolan, 1986, Medicine Lake, California; Beard, 1986, compilation from different sites; Yagi & Takeshita, 1987, Japan; Fichaut et al., 1989, Martinica, Lesser Antilles; Beard & Borgia, 1989, Arenal volcano, Costa Rica; Heliker, 1995, Mt. St. Helens; Hickey-Vargas et al., 1995, Calbuco volcano, southern Chile; gabbroic plutons: Smith et al., 1983, Peninsular Ranges batholith, California; Ulmer et al., 1983, Adamello batholith, Italy; Fabriès et al., 1984, Saint Quay-Portrieux intrusion, Britanny, France; Otten, 1984, Artfjället gabbro, Swedish Caledonides; Regan, 1985, Coastal batholith of Peru; Whalen, 1985, Uasilau-Yau Yau Intrusive Complex, New Britain; Beard, 1986, compilation from different sites; Himmelberg et al., 1987, Yakobi intrusion, Alaska; Beard & Day, 1988, Smartville Complex, California; DeBari & Coleman, 1989, Tonsina Complex, Alaska; Springer, 1989, Pine Hill Complex, California; Kepezhinskas et al., 1993, Kamchatka; DeBari, 1994, Fiambalá intrusion, Argentina; Tepper, 1996, Chilliwack batholith, Washington; Sisson et al., 1996, Hornblende gabbro sill, California). This is in marked contrast with the rare occurrences of hornblende phenocrysts in basalts or basaltic andesites in arc volcanoes world wide (Sigurdsson & Shepherd, 1974, Kick'em-Jenny Volcano, Lesser Antilles; Arculus, 1976, Grenada, Lesser Antilles; Arculus et al., 1976, Bogoslof Volcano, Alaska; Luhr & Carmichael, 1985, Cerro la Pilita, Mexico; Peterson & Rose, 1985, Ayarza caldera, Guatemala; Rose, 1987, Santa María Volcano, Guatemala). The occurrence and compositions of hornblende (e.g., high mg-number and Cr2O3 contents) in gabbroic xenoliths has led to some authors (e.g., Conrad & Kay, 1984; Yagi & Takeshita, 1987; Beard & Borgia, 1989) to propose that it was an early crystallizing mineral, with the implication that it might be responsible for the calc-alkaline trend of subduction zone magmas (e.g., Yagi & Takeshita, 1987). Experiments on basaltic and basaltic andesitic water-bearing compositions have shown that, in some instances, hornblende can be a near liquidus mineral even at low pressures (< 3 kbar; e.g., Sisson & Grove, 1993; Barclay & Carmichael, in prep). This has led to the interpretation that the paucity of hornblende phenocrysts in mafic lavas is due to an increase in magma crystallinity once hornblende crystallizes, thus hornblende-bearing mafic magmas are unable to erupt (Barclay & Carmichael, in prep). The petrological, mineralogical and geochemical characteristics of the San Pedro xenoliths suggest that the large amount of hornblende in gabbroic rocks can be due to open-system percolation in crystal piles. Aside from the possibility of hornblende crystallizing due to protracted closed-system crystallization, we propose that migration of evolved melt (and fluid) in plutonic systems can produce reactions with early crystallized minerals (olivine, Cr-spinel, pyroxenes and plagioclase) leading to the production of large proportions of hornblende (and phlogopite) with compositions that resemble those of early crystallization from mafic magmas (e.g., high mg-numbers and Cr2O3 contents).
Conclusions
A detailed petrographic and compositional study of two groups of gabbroic xenoliths from the Tatara-San Pedro Complex has shown that these xenoliths record multistage crystallization histories involving reactions related to melt migration. Reaction of mafic cumulus minerals (Cr-spinel, olivine, pyroxenes and plagioclase) with percolating evolved melts and fluids triggered crystallization of hornblende and phlogopite. We suggest that the higher abundance of these hydrous minerals in calc-alkaline gabbros compared to basalts or basaltic andesites can be explained by melt migration and reaction processes within the cumulate piles of magma chambers.
Acknowledgements
I would like to thank: A.Wulff and M.Rhodes for the XRF analyses, P. Voldet for the ICP-AES analyses, F. Ramos for the TIMS analyses, Y.Vinzce and T.Thon-That for the 40Ar/39Ar analyses, and F. Parat for the electron microprobe analyses of apatite. I'm also grateful to J. Barclay for making me available her unpublished experimental data and for many discussions about occurrences of horblende-bearing mafic lavas. Field work was funded by the ASSN.
Appendix
Electron microprobe analyses
Analyses were performed with a Cameca SX-50 at the University of Lausanne. Analyses were carried out using WDS, with an operating voltage of 15 kV. Beam current was 7 nA for glass, 15nA for plagioclase, hornblende, phlogopite, 20 nA for olivine and pyroxenes, 25 for spinel and ilmenite, and 30 nA for apatite. Beam diameter was ~ 1 µm, except for phlogopite (~2 µm), and for glass (5-10 µm).
X-ray Fluorescence analyses
Major and trace elements were determined at the University of Massachusetts by standard techniques (see Rhodes, 1988). The 2-s relative precisions are as follows: SiO2: 0.6%, TiO2: 0.5%, Al2O3: 1%, Fe2O3: 0.5%, MnO: 6%, MgO: 1.2%, CaO: 0.6%, Na2O: 5 %, K2O: 1.5%, P2O5: 4.5%. Rb: 10%, Sr: 1%, Zr: 1%, Nb: 6%, La: 4%, Ce: 12%, Y: 2.5%, V: 2%, Cr: 1.8%, Ni: 2%, Zn: 1%.
Inductively coupled plasma-atomic emission spectrometry analyses
Rare earth element analyses were performed at the University of Geneva. Details of the methods used are found in Voldet (1993). The relative 2-s precisions range between 5-10 % depending on the concentration of the element. The REE concentrations determined by INNA (University of Massachusetts) and by ICP-AES agree within 10% for most elements (see text).

Thermal ionization mass spectrometry analyses
Analyses were made ate the University of California at Los Angeles. The 2-s relative precisions for Rb and Sr are ~1 %. The methods used are described in Ramos (1992). Blanks were in the range of 10 ppb for Rb and less than 1 ppb for Sr.
III. Glass-Bearing gabbroic xenoliths from volcán San Pedro (36o S), Chilean Andes: evidence of melt migration and reaction fractionation mechanisms from trace element zoning of plagioclase and glass
Abstract
A late Holocene eruption of Volcán San Pedro (Tatara-San Pedro Volcanic Complex, TSPC, 36o S, Chilean Andes) brought to the surface a suite of gabbroic xenoliths. The xenoliths consist of Cr-spinel, olivine, minor clinopyroxene, orthopyroxene, hornblende, plagioclase, and phlogopite forming a crystal network with SiO2-rich (67-72 wt %) glass filling the interstices. Glass is distributed in pockets bounded by euhedral crystal faces suggesting that it is residual and not due to partial melting, thus we interpret these xenoliths as fragments of a partially crystallized zone of a magma chamber. This study combines textural, and major element plagioclase compositions determined by the electron microprobe plus trace element compositions determined by ion microprobe (Mg, Fe, Ti, K, Rb, Sr, Ba, La, Ce, Y, and Li), together with the composition of the interstitial glass to evaluate the differentiation mechanisms recorded in these xenoliths. Plagioclase crystals not included in hornblende, orthopyroxene, and phlogopite are characterized by anortithe-rich cores (An86-70) mantled by more sodic rims (An40-20). Cores and rims are separated by a compositional gap of 30-40 mol % anorthite. Concentrations of Fe, Ti, K, Sr, Ba, La, Ce follow this abrupt major element shift suggesting a major change in the composition of the interstitial melt. Calculated melt compositions in equilibrium with plagioclase are consistent with an early episode of differentiation from basaltic to basaltic andesitic liquids, after which the interstitial melt changed abruptly to a dacitic composition. This is explained by an event of melt migration, where the interstitial mafic liquid was displaced by a dacitic melt that reacted with early-crystallized mafic minerals producing crystallization of orthopyroxene, hornblende, and phlogopite. During and after this event, the interstitial melt evolved to rhyolitic compositions. However, the calculated compositions in equilibrium with plagioclase rims are unlike the interstitial rhyolitic glass of the xenoliths or unlike dacitic to rhyolitic lavas and plutons found at the TSPC. This could be due to the complex nature of the melt migration and reaction processes that may occur in partially crystallized zones of magma chambers, or to uncertainties in the plagioclase-melt partition coefficients in silicic magmas.
1. Introduction
The physical and chemical processes that occur in partially crystallized zones of magma chambers have been treated experimentally (Chen & Turner, 1980; Turner & Gustafson, 1981; McBirney et al., 1985; Huppert et al., 1986; Bédard et al., 1992; Tait & Jaupart, 1992; Hort et al., 1999), and theoretically (Kerr & Tait, 1986; Brandeis & Jaupart, 1987; Martin et al., 1987; McKenzie, 1987; Lesher & Walker, 1988; Marsh, 1995; Spera et al., 1995; Hort et al., 1999). An important result of these studies is that evolved interstitial liquids of partially solidified zones may migrate within crystal-mush zones (either by convection or by compaction) and the interior of the chamber. The main geological evidence for such processes is found in layered intrusions where expulsion and replenishment of interstitial liquids, locally accompanied by replacement reactions, is recognized as an important differentiation mechanism (e.g., Muskox, Irvine, 1980; Skaergaard, McBirney, 1995; Bushveld, Mathez, 1995; Stillwater Complex, Meurer et al., 1997). Where such intrusions have undergone long term cooling and subsolidus reequilibration, post-solidification processes may partially obscure the compositional and petrographical effects of melt migration. Rare, partially solidified xenoliths that have been sampled naturally from active subvolcanic magma reservoirs provide another source of geological information to study the physical and chemical processes that occur in partially crystallized zones of magma chambers (e.g., Hermes & Cornell, 1981; Tait, 1988; deSilva, 1989, Turbeville, 1992; Widom et al., 1993; Brophy et al., 1996). Although such xenoliths offer no information about the geometry or scale of zones in which differentiation processes may have operated, they preserve microscopic textural and compositional features that have been obscured or even erased in surface outcrops of large gabbroic intrusions.
A late Holocene dacitic lava flow of Volcán San Pedro contains a suite of glass-bearing gabbroic xenoliths. This study uses textures plus major and trace element zoning of plagioclase together with the trace element composition of interstitial glass and hornblende to evaluate the differentiation mechanisms recorded by these xenoliths. Brophy et al. (1996) in a study of plagioclase textures, major and trace element zoning in a suite of glass-bearing gabbroic xenoliths from Medicine Lake Volcano, documented the fractionation mechanisms that occurred during closed-system evolution of a partially crystallized zone of a magma chamber. The study of the San Pedro xenoliths reveals a more complex scenario involving migration of interstitial liquids and reactions with the crystal-network through which these melts percolated. Specifically, we will test the hypothesis presented in Chapter II that the mafic intercumulus liquid in equilibrium with olivine, clinopyroxene, Cr-spinel, and anorthitic plagioclase, was displaced by a dacitic liquid that reacted with the mafic cumulus minerals to produce post-cumulus orthopyroxene, hornblende, and phlogopite.
2. Geological setting
Holocene Volcán San Pedro is the youngest and most prominent volcanic edifice (3621 m) of the larger Quaternary Tatara-San Pedro Complex (~ 55 km3; TSPC), which is located on the volcanic front of the Southern Volcanic Zone (SVZ) of the Andes, at 36o S, 71o51'W (Singer et al., 1997; see Figs. 1 and 2 of Chapter II). The magmatic activity at Volcán San Pedro is divided into a cone-building phase comprising andesitic and dacitic lavas, and a younger phase that post-dates the sector collapse of the eastern flank of the volcano, which was accompanied by an explosive eruption that produced air-fall dacitic deposits (Singer & Dungan, 1992). This was followed by the eruption of a sequence of lava flows that apparently record the downward tapping of a strongly zoned magma chamber. The eruptive sequence comprises: (1) 0.2 km3 of biotite-hornblende dacite (66 wt % SiO2) containing abundant gabbroic xenoliths (up to 45 cm in diameter) and quenched mafic inclusions (QMI), (2) 0.5 km3 of two-pyroxene dacite (63 wt % SiO2) with abundant QMI, and (3) 0.1 km3 of two-pyroxene andesite (61 wt % SiO2) with rare QMI. The last volcanic activity consisted of 0.2 km3 of basaltic andesite magma (55-57 wt % SiO2) which rebuilt the summit cone. The fact that the xenoliths have been found exclusively in the first lava following a sector collapse and the ensuing explosive eruption suggests that they are fragments of the conduits or upper parts of the margin of the San Pedro magma chamber which were shattered and incorporated during the eruption (in a similar fashion to the May 18, 1980 Mount St. Helens eruption; Heliker, 1995).
Previous studies (Chapter II) distinguished between two suites of gabbroic xenoliths, but in this chapter we are only concerned with the suite of melanorites and norites bearing interstitial glass (Group I of Chapter II) which are potentially co-magmatic with the Holocene volcanism.
3. Petrography and crystallization sequence of the xenoliths
These xenoliths are olivine norites to melanorites (classification following Streckeisen, 1976; Le Maitre, 1989; modes in Table 1 of Chapter II), with SiO2 contents of 46-47 wt %, MgO of 20-21 wt %, and K2O of 0.52-0.71 wt %. For a detailed discussion of the major, minor and trace element composition of the xenoliths see Chapter II.
The xenoliths are characterized by the presence of interstitial, mainly rhyolitic glass (0.5 to 13 vol. %) distributed in pockets and bounded by euhedral crystal faces, suggesting that these glass pockets correspond to residual liquid and that it is not due to partial melting. Cumulus olivine, clinopyroxene, and Cr-spinel are commonly anhedral and resorbed. In contrast, post-cumulus orthopyroxene, hornblende, and phlogopite are subhedral to euhedral and include resorbed olivine, clinopyroxene, and Cr-spinel (Fig. 1). Plagioclase is euhedral and occurs as inclusions in orthopyroxene, hornblende, and phlogopite, and in the matrix glass (Fig 1). Minor euhedral apatite is also present in the glass. From textural relations we infer reaction relations between liquid and olivine + clinopyroxene + Cr-spinel to produce orthopyroxene + hornblende + phlogopite. For a more detailed textural and mineralogical description refer to Chapter II.
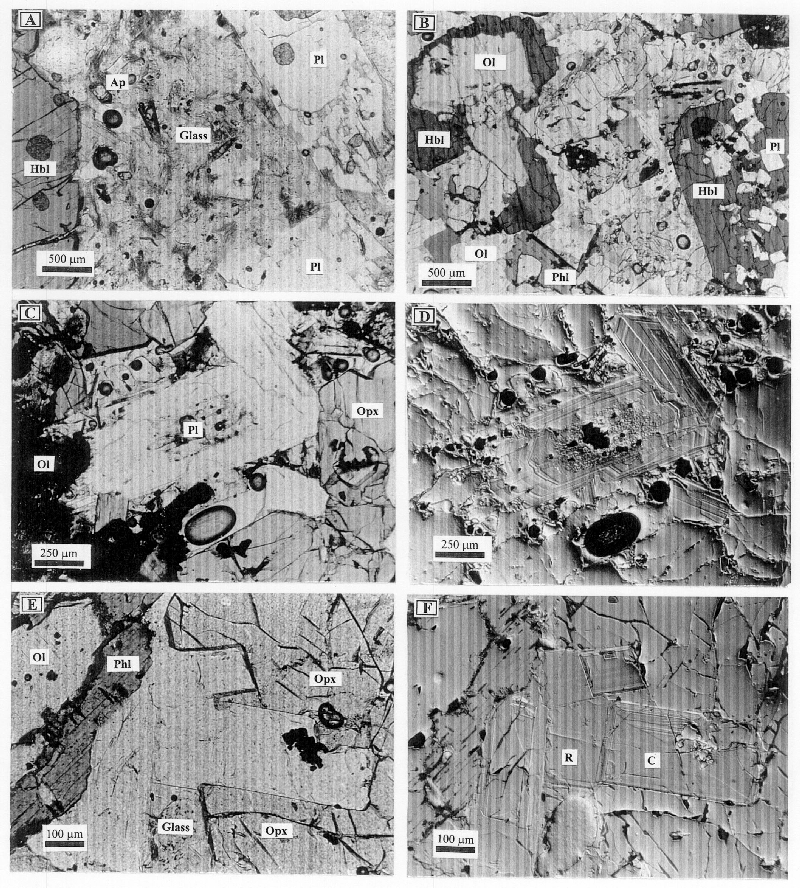 Figure 1. Photomicrographs of the textures of the xenoliths.
Figure 1. Photomicrographs of the textures of the xenoliths.
A. Interstitial glass with apatite (Ap) crystals. Note the euhedral crystal faces of hornblende (Hbl) and plagioclase (Pl). B. Reaction textures of sample Hx14n. Hornblende and phlogopite (Phl) are surrounding anhedral olivine (Ol) suggesting a reaction relation. In contrast, note that plagioclases are euhedral. C. Sample Hx14b. Euhedral plagioclase surrounded by vesiculated interstitial glass. D. NDIC image of the previous photomicrograph. Note the numerous oscillatory zones and the complex core of the plagioclases. E. Sample Hx14n. Plagioclase crystals partially enclosed by orthopyroxene (Opx). F. NDIC image of the previous photomicrograph. Plagioclases are sector zoned, but with scarce oscillatory zones or dissolution surfaces. The plagioclase cores (C) and rims (R) can be clearly distinguished.
As previously discussed (Chapter II) the crystallization history of the xenoliths is characterized by the following magmatic events: (1) crystallization and accumulation of olivine (± Cr-spinel), followed by crystallization of clinopyroxene and anorthitic plagioclase (An86-An70), (2) displacement of the mafic interstitial liquid by an evolved melt (e.g., dacitic), and (3) reaction of the dacitic liquid with olivine + Cr-spinel + clinopyroxene to produce orthopyroxene + hornblende + phlogopite, followed or accompanied by further crystallization of An-poor plagioclase (e.g., An45-An6) and apatite.
4. Major and trace element composition of hornblende and glass
Major and minor element analyses of hornblende and glass were obtained with an electron microprobe (Cameca SX-50, University of Lausanne), and trace and minor element analyses were obtained with an ion microprobe (Cameca IMS-4f, University of Edinburgh). We performed six ion microprobe analyses in two hornblende crystals of sample Hx14n. The following elements were measured: Ti, Rb, Sr, Ba, Zr, Nb, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Yb, and Li (Table 1). Seven ion microprobe analyses including Mg, Ti, Rb, Sr, Ba, Y, La, Ce, Sm, and Li were performed on the interstitial glass of sample Hx14b, and one in sample Hx14n (Table 1). Unless otherwise noted, the 2-s relative precision of the discussed elements is better than 5 %. Analytical details are presented in Appendix I.
 Table 1. Major and trace element compositions of hornblende and glass.
Table 1. Major and trace element compositions of hornblende and glass.
4.1. Hornblende
Hornblende is magnesiohastingsite (classification after Leake et al., 1997), with Cr2O3 contents commonly <0.30 wt %, but occasionally up to 1 wt %. Center to margin compositional traverses show that TiO2 concentration decreases (from ~ 4 wt % to ~1 wt %) whereas Al2O3, MgO, and Na2O abundances increase slightly towards the crystal margin.
Some crystals are sector zoned and important compositional differences in Ti, Al, Mg and Ca occur across sectors. The two hornblende crystals have overlapping trace element compositions, but they display rather large concentration ranges. Rb varies from 7.4 to 8.3 ppm, Sr from 171-225 ppm, Ba from 57 to 74 ppm, La from 3.5 to 6.6 ppm, and Y from 31 to 45 ppm (Table 1).
4.2. Interstitial glass
The interstitial glass is vesiculated and is variable in composition. Glass from sample Hx14n ranges from dacitic to rhyolitic, with SiO2 varying from 66.7 to 71.6 wt %, Al2O3 from 18.7 to 14.5 wt %, Na2O from 2.6 to 6.6 wt %, and K2O from 3.7 to 8.6 wt %. Glass from sample Hx14b is rhyolitic, with higher SiO2 (69 to 75 wt %), similar Al2O3 (14-17 wt %), but lower Na2O (3.2 to 5.3 wt %) and K2O (3.6 to 5.5 wt %) contents (Table 1; additional glass analyses can be found in Chapter II).
The high MgO concentration (0.8-1.4 wt %) of analyses b-3 and b-8 compared to the rest of the glasses (0.17-0.32 wt %) and to the electron microprobe analyses suggest that they are mixtures of glass and minerals, and thus they will not be further considered. The trace element composition of glass in sample Hx14b is variable, with large concentration ranges in Ba (252-464 ppm), Rb (96-139 ppm), and Sr (6-9 ppm). The concentrations of La (20-24 ppm), Ce (41-45 ppm), and Sm (1.6-2 ppm) are more homogeneous. The single glass analysis from sample Hx14n has higher Sr (46 ppm) and Ba (588 ppm) concentrations, lower Ce (34 ppm) and Sm (1.1 ppm) than glass of sample Hx14b.
5. Textures and major element zoning of plagioclase
Center to margin electron microprobe analyses were performed across 42 plagioclase crystals in samples Hx14b and Hx14n, with a total of ~ 2500 analyses. Plagioclase textural features were examined with Nomarski Differential Interference Contrast (NDIC), following the methods described in Singer et al. (1995). Plagioclases of both samples are strongly zoned, with cores at An86 and rims at ~ An20. Because plagioclase textures and certain zoning patterns among the two samples are different, they will be described separately. For the following description and discussion, plagioclase core compositions are those of anorthite contents between An86 to An70 mol %, compositions in transition zones between cores and rims are those of An70 to An45, and rim compositions are those with <An45.
Plagioclase crystals in sample Hx14n are almost featureless, with only weak oscillatory zoning and few dissolution surfaces (Fig. 2a-d). Normally zoned euhedral cores (An86 to An75) mantled by normally zoned rims (An45 to An30) characterize plagioclase crystals that are not included in orthopyroxene, hornblende, or phlogopite (Fig. 2a-d). Plagioclase cores and rims are comparable in width, and are separated by a compositional gap of 30 to 35 mol % An. This abrupt compositional shift typically occurs over a distance of less than 100 µm, and it results in a bimodal compositional distribution with one mode at An80-82 and another at An40-42 (Fig. 3). Plagioclase crystals included in orthopyroxene, hornblende, or phlogopite have nearly identical compositions and are normally zoned from An86 to An40, (most compositions between An86 and An75; Fig. 3), suggesting that the three post-cumulus minerals crystallized concurrently.
Plagioclase crystals in sample Hx14b display more complex textural and compositional features such as oscillatory zoning, dissolution surfaces and mineral and melt inclusions (Fig. 2e-f). Plagioclase crystals not included in orthopyroxene, hornblende, or phlogopite, show normally zoned cores from An86 to An70 that are mantled by normally zoned rims with anorthite contents as low as An6 at the edge of some crystals (Fig. 3). Typically, cores (~ 500 µm) are much larger than rims (~ 50 µm) and are separated by a narrow transition zone (~50 µm) in which decreases of up to 40 mol % An occur. Plagioclase crystals included in orthopyroxene are also normally zoned from An86 to An42, but most compositions are between An85 and An70 (Fig. 3). Multiple minor anorthite shifts (<10 mol %) associated with dissolution surfaces are present in many plagioclase cores. These secondary features will not be considered in detail, as we are mostly interested in the large compositional differences between plagioclase cores and rims.
Despite the similar post-cumulus histories in which hornblende, orthopyroxene, and phlogopite were produced by late reactions, plagioclase cores of sample Hx14b show numerous oscillatory zones and dissolution surfaces whereas those of sample Hx14n do not. This implies that plagioclases from the two samples may have resided in different magmatic environments prior to being fixed in the cumulus framework. Specifically, it would appear that the numerous oscillatory zones and dissolution surfaces observed in plagioclases of sample Hx14b are characteristic of plagioclase phenocrysts of the interior of a convecting magma chamber (Singer et al., 1995; Hattory & Sato, 1996), whereas the featureless plagioclases of sample Hx14n have been interpreted as evidence of crystallization in a more static environment, such as the partially crystallized zone of a magma chamber (e.g., Brophy et al., 1996). We envision a scenario where plagioclase crystals of sample Hx14b were phenocrysts from the interior of a convecting magma chamber which became captured by its crystallizing margin (sample Hx14n). Such a process has been previously proposed by Loomis & Welber (1982) and by Kuritani (1998) to explain the zoning profiles of plagioclase from plutonic and volcanic environments.
 Figure 2. NDIC images and anorthite mol % profiles of plagioclase.
Figure 2. NDIC images and anorthite mol % profiles of plagioclase.
Distance in micrometers is from the margin. A. Sample Hx14n. Plagioclase (Pl2n) consists of an euhedral normally zoned core with scarce oscillatory zones and dissolution surfaces. An almost featureless rim mantles the core. Cores and rims are separated by a composition gap of ~ 35 mol % An that occurs in ~ 15 µm. The electron microprobe traverse is marked by the small rounded pits, whereas as the larger pits are the ion microprobe analyses. B. Sample Hx14n. Plagioclase (Pl4n) is four times smaller than the previous crystal but it shows nearly identical textural and compositional features. C. Sample Hx14n. Plagioclase consisting of an absolutely euhedral sector zoned core of virtually constant anorthite composition. Dissolution surfaces and oscillatory zones are only present right before the rim. The rim is normally zoned and lacks dissolution surfaces or oscillatory zones. D. Sample Hx14n. Plagioclase included in hornblende is normally zoned from An83 in the center to An50 in the margin. Oscillatory zones or dissolution surfaces are only present in the crystal margin where plagioclase composition changes abruptly (~ 50 µm). E. Sample Hx14b. Plagioclase included in hornblende. Note the numerous oscillatory zones and the complex core is very different from the plagioclase crystal of sample Hx14n (Fig.2D). Black thick line marks the position of the electron microprobe traverse. F. Sample Hx14b. Normally zoned plagioclase in contact with interstitial glass. Note the extreme change in anorthite compositions at the last 50 µm. The complex zoning texture with numerous oscillatory zones and dissolution surfaces is very different from plagioclases of sample Hx14n. Black thick line marks the position of the electron microprobe traverse.
 Figure 3. Histogram of plagioclase compositions (total of 42 crystals).
Figure 3. Histogram of plagioclase compositions (total of 42 crystals).
Plagioclases not included in Opx, Hbl or Phl (a and b) show a large compositional spectra. The bimodal plagioclase composition distribution of sample Hx14n (b) is due to the compositional gap between cores and rims. Plagioclases included in Opx, Hbl and Phl (c and d) display narrower compositional spectra.
6. Trace element zoning of plagioclase
Cores to rim ion microprobe traverses were performed on nine representative plagioclase crystals. These include six plagioclase crystals displaying a large anorthite compositional gap (Pl2n, Pl3n, Pl4n, Pl7n, Pl4b, and Pl5b), and three plagioclase crystals included in hornblende of sample Hx14n (Pl6n, Pl1n and Pl5n). In total, 90 ion microprobe analyses were performed and the following elements were measured: Ca, Mg, Fe, Ti, K, Rb, Sr, Ba, La, Ce, Y, and Li (Table 2). Trace element compositions of plagioclase rims were determined only in plagioclases of sample Hx14n, as plagioclase rims in sample Hx14b are too narrow. Comparison between electron microprobe and ion microprobe concentrations of K, Ba, and Mg agreed within less than 10% (see Appendix I). The 2-s relative precision of all elements discussed is better than 5%, except for La, Ce, and Li in plagioclase cores (10-15%), and for all Rb (25-33%), and Y (24%) analyses. Analytical details of the ion microprobe analyses are presented in Appendix I.
6.1. Plagioclase cores (An86 to An70)
Concentrations of Fe, Ti, Sr, Ba, K, Rb, La, Ce, and Y in plagioclase cores are comparable among the two samples and among different crystals, whereas Mg and Li are highly variable. Concentrations of Ti, Sr, Ba, La, Ce, and Li tend to increase towards the margins of cores (Figs. 4 and 5). Iron concentrations stay more or less constant, whereas K increases in some crystals (Pl4n, Pl7n and Pl4b) and decreases in others (Pl2n and Pl5b). The Mg concentration always decreases. The concentration profiles of Rb and Y (not shown) do not display well-defined enrichment or depletion trends, probably due to the large analytical errors (25-30%). The increasing Ti, Sr, Ba, K, La, and Ce concentrations coupled with decreasing Mg (although see Discussion) can be explained by the crystallization of a mineral assemblage consisting of olivine, clinopyroxene, and anorthitic plagioclase (An85-70) from a basaltic magma. The concentrations of Mg, Ti, Sr, Ba, and K of plagioclase cores are similar to those measured for the anorthite-rich xenocrysts (An82-76) in the host dacite (Fig. 6), which were also inferred to have crystallized from basaltic magmas (Singer et al., 1995).
6.2. Transition zones between cores and rims (An70 to An45)
Large and abrupt changes in Fe, Ti, Sr, Ba, K, La, Ce, and Y concentrations occur at the boundaries between cores and rims, and mimic abrupt changes in major elements. Rims have higher concentrations of Sr, Ba, K, La, Ce and lower concentrations of Fe, Ti, and Y than cores (Figs. 4 and 5). Enrichment factors between rims (or transition zone) and cores are 1.5-2.1 for Sr, 2.5-4.8 for Ba, 2-4.5 for K, 2.2-7.3 for La, and 1.9-5.5 for Ce, and depletion factors are 1.2-2.6 for Fe, and 1.5-10.6 for Ti. The Li and Rb concentrations do not change significantly between cores and rims. It is worth noting that the Mg, Ti, Sr, Ba, and K concentrations in the plagioclase phenocrysts (An64-46; Singer et al., 1995) of the host dacite lava are between those of plagioclase cores and rims, and partly overlap with compositions of the transition zone (Fig. 6). This suggests that plagioclase with compositions transitional between cores and rims crystallized from melts of a composition comparable to that of the host dacite. The large Ti and Fe concentration differences between cores and rims might reflect concomitant crystallization of hornblende and orthopyroxene, as no Fe-Ti oxides are present in the xenoliths.
6.3. Plagioclase rims (<An45)
The Mg, Fe, Ti, La, and Ce concentrations of plagioclase rims remain more or less constant from the inner to outer rim of all crystals (Fig. 4). The concentration profiles of Sr, Ba, and K are more complex. Plagioclase crystal Pl2n shows two Ba and Sr concentrations lows associated with two K concentration peaks, which in turn are related to small (<10 mol %) anorthite shifts. In general, however, Sr and Ba concentrations tend to decrease towards the outer part of the rim, whereas K concentrations increase. The decreasing rim-ward Sr and Ba concentrations can be explained, to a first approximation, by crystallization of a mineral assemblage consisting of low anorthite plagioclase (e.g., <An45) and phlogopite.
 Figure 4. Anorthite mol % and trace element profiles of selected plagioclase crystals of sample Hx14n.
Figure 4. Anorthite mol % and trace element profiles of selected plagioclase crystals of sample Hx14n.
Gray band indicates the plagioclase composition of the transition zone. The size of the symbol is similar to or larger than the 2-s precision of all elements.
 Figure 5. Anorthite mol % and trace element profiles of two plagioclase crystals of sample Hx14b.
Figure 5. Anorthite mol % and trace element profiles of two plagioclase crystals of sample Hx14b.
Gray band indicates the plagioclase composition of the transition zone. The size of the symbol is similar to or larger than the 2-s precision of all elements.
The higher K content of the plagioclase rims compared to the plagioclase phenocrysts of the host dacite suggests that plagioclase rims crystallized from more evolved melts than the dacite (Fig. 6).
 Figure 6. Variation diagrams of trace element composition of plagioclase.
Figure 6. Variation diagrams of trace element composition of plagioclase.
Also shown are the compositions of the plagioclase phenocryst and xenocryst of the dacite lava in which the xenoliths were found (excluding four outermost rim analyses of the plagioclase phenocryst, see Singer et al., 1995). The size of the symbol is similar to or larger than the 2-s precision of all elements. See text for discussion.
7. Discussion
7.1. Origin of the plagioclase compositional gap
The An mol % of plagioclase depends not only on magma composition but also on its temperature, pressure, and water content (e.g., Yoder, 1969; Housh & Luhr, 1991; Nelson & Montana, 1992; Sisson & Grove, 1993). Specifically, significant changes in the water pressure can induce shifts in the plagioclase of up to 20 An mol % (e.g., Panjasawatwong et al., 1995). However, the coupled major and trace element variations displayed by the plagioclases in these xenoliths suggest that the large anorthite shift is mainly due to an abrupt change in the composition of the melt rather than to changes in the water content.
A mechanism capable of producing large and rapid compositional changes in calc-alkaline magmas was proposed by Grove & Donnelly-Nolan (1986) in a study of silicic lavas, andesite inclusions and gabbroic xenoliths of Medicine Lake Volcano. Specifically, they suggested that closed-system peritectic reactions that involve liquid, olivine and clinopyroxene as reactants, and orthopyroxene and hornblende as products could lead to large compositional changes in the melt. Due to the shallow slope in temperature-composition diagrams of such reactions, sudden increases in the crystallinity of the magma and large changes in the liquid composition can occur over small temperature intervals (Grove & Donnelly-Nolan, 1986). As the San Pedro xenoliths display the same reactions involving hornblende and orthopyroxene (but also phlogopite) to the Medicine Lake gabbroic xenoliths, it seems plausible that a brusque and large amount of hornblende crystallization could produce rapid and major compositional changes in the liquid (particularly lowering the Ca/Na). However, Brophy et al. (1996) investigated plagioclase zoning patterns in several Medicine Lake gabbroic xenoliths and found smooth, monotonic trends of decreasing An mol % (An74-30) instead of the abrupt zoning patterns that would be expected from a sudden crystallization of hornblende and orthopyroxene. The plagioclase trace element traverses also display smooth zoning profiles, with the onset of hornblende and orthopyroxene (and Fe-Ti oxides) crystallization being marked by a change in the slope of the Fe and Ti plagioclase concentrations rather than by an abrupt change. Brophy et al. (1996) interpreted these profiles as a record of progressive closed-system crystallization from andesitic to rhyolitic compositions. We take this as evidence that simple, closed-system crystallization involving orthopyroxene and hornblende does not explain the extreme and abrupt plagioclase zoning present in the San Pedro xenoliths, and hence another interpretation is required.
In Chapter II, we suggested that the mafic intercumulus liquid in equilibrium with olivine, clinopyroxene, Cr-spinel, and plagioclase cores, was displaced through porous flow by a dacitic liquid that reacted with the mafic cumulus minerals to produce post-cumulus orthopyroxene, hornblende, and phlogopite. In such a scenario, the abrupt and large compositional gap displayed by the plagioclase can be explained by: (1) the much lower Ca/Na values of the dacitic liquid compared to the displaced mafic liquid, and (2) the crystallization of hornblende which further decreased the Ca/Na values of the interstitial melt from which plagioclase rims crystallized. The abrupt major element shift from cores to rims, and the minimal occurrence of plagioclase of intermediate compositions (e.g., An60), support the idea that melt displacement occurred as a wave or a pulse without significant mixing between the two melts, consistent with their presumably large viscosity difference.
The trace element concentration differences between cores and rims provide additional support for the melt migration event. Enrichment factors between cores and rims range from 2.2 for K, to 7.3 for La, and depletion factors are as large as 10.6 for Ti (Fig. 6). Such large compositional changes are difficult to reconcile with closed-system evolution. To further investigate the proposed crystallization sequence and the origin of the plagioclase compositional gap, in the following section we have calculated the liquids in equilibrium with the plagioclase composition.
7.2. Some considerations prior to reconstruction the liquid composition in equilibrium with plagioclase
Using the melt composition in equilibrium with plagioclase to model magmatic processes has been successfully applied by several authors to trace magmatic processes (Blundy & Shimizu, 1991; Singer et al., 1995; Brophy et al., 1996; Papike et al., 1997; Bindeman et al., 1999). Nevertheless, the validity of such approach relies on several assumptions: (1) diffusion has not modified the plagioclase composition after it crystallized, (2) appropriate plagioclase-melt partition coefficients are available, and (3) plagioclase crystallized under local equilibrium conditions. The first two assumptions are discussed below. The third can only be addressed by calculating the equilibrium melts.
7.2.1. Diffusion in plagioclase
Several observations suggest that Mg diffusion has occurred in some plagioclase crystals. First, Mg is the only element that does not show an abrupt concentration change between cores and rims (Figs. 4 and 5). This is neither consistent with the melt migration hypothesis nor with a sudden and large amount of crystallization. We interpret this as evidence that Mg exchange between plagioclase cores and rims has occurred by diffusion within the plagioclase. Second, the fact that small plagioclase crystals (< 200 µm) with high anorthite contents (e.g., Pl3n and Pl4n) have maximum Mg concentrations that are much lower than those of large crystals (e.g., Pl2n and Pl7n) could also be explained by Mg diffusion within the plagioclase or between plagioclase and melt.
That the plagioclase Mg concentration might have been affected by diffusion is supported by the recent work of LaTourrette & Wasserburg (1998), who found that the rate of Mg self-diffusion in anorthite is very high. In fact, Mg diffusion in anorthite is as fast as Mg diffusion in forsteritic olivine (Chakraborty, 1997), for which evidence of reequilibration is well documented in the literature (e.g., Lambert & Simmons, 1987). The rest of the analyzed elements vary in a stepwise pattern across the plagioclase compositional gap, in accord with much lower diffusion rate estimates for these elements.
For example, at 900oC, the diffusion coefficient of Mg (dMg) in anorthite is 3.5-5* 10-19 m2/s, which (for the same temperature) is approximately two orders of magnitude larger than dSr in anorthite (Giletti & Casserly, 1994), almost three orders of magnitude larger than the diffusion coefficient of the rare earth elements (e.g., Nd; Giletti, 1997), and almost three orders of magnitude larger than the NaSi-CaAl interdiffusion (in water-bearing systems, Liu & Yund, 1992). In contrast, Li has a diffusion coefficient five to ten orders of magnitude larger than any other analyzed element (Giletti & Shanahan, 1997). Accordingly, we take the analyzed Mg concentration in the core as the minimum concentration, whereas the rest of the elements (except Li) have probably not been affected by diffusion, and thus can be used to constrain magmatic processes.
7.2.2. Plagioclase-melt partition coefficients
Plagioclase-melt partition coefficients exist for numerous elements and for a broad range of plagioclase and melt compositions (see compilations by Blundy & Wood, 1991, Green, 1994; Bindeman et al., 1998; R. Nielsen at http://www-ep.es.llnl.gov/germ/ partitioning.html). Nonetheless, the partition coefficient values for any particular element may vary widely among different data sources, even for comparable plagioclase and melt compositions. Recently, Blundy & Wood (1991 and 1994) and Bindeman et al. (1998) have developed empirical equations that relate the partition coefficient to plagioclase composition and temperature, which given the large compositional ranges of the studied plagioclases seem the most pertinent to use. For Sr and Ba we used the equations of Blundy & Wood (1991; equations #18 and #19), and for Mg, Ti, Fe, K, La, and Ce we used the equations of Bindeman et al., (1998; their Table 4, at natural concentration levels). The expressions that relate the partition coefficient with the plagioclase composition and temperature take the form:

where R is the gas constant, T is temperature, D is the partition coefficient, XAn is the anorthite mol fraction, and a and b are the regressed parameters that vary for every element. We have estimated the error associated with the partition coefficients determined by using equation (1) by error propagation (assuming independent errors; Taylor, 1982) of the uncertainties in the regressed parameters. We did not take into account the error in An mol % determination from the electron microprobe analyses, the error of the ion microprobe analyses, or the uncertainties in the temperature estimations (the derived equation can be found in Appendix II). At temperatures between 950oC and 1150oC and plagioclase compositions between An80 and An40, the 2-s relative errors of the partition coefficients vary from 14% to 20% for Sr, Mg, and K, and from 22% to 52% for Ti, Fe, Ba, La, and Ce.
In general, partition coefficients determined with equation (1) are not highly temperature dependent. Changing the temperature by 100oC varies the partition coefficient by less than 10% for most elements, except for Sr that varies by 15%.
Temperatures of 1150oC and 950oC have been estimated, respectively, for the basaltic and dacitic magmas of Volcán San Pedro (Singer et al., 1995). Accordingly, we have used a temperature of 1150oC to calculate the melts in equilibrium with plagioclase cores, 950oC for the rims, and 1050oC for the transition zone compositions.
7.3. Composition of the calculated melts
In general, calculated melts in equilibrium with plagioclase show progressive compositional variations from the inner to outer cores and from the inner to rims (not shown). This means that the geochemical trends displayed by the calculated melts in variation diagrams can be roughly interpreted as reflecting the successive compositional changes that occurred in the liquid. The compositional variations of the calculated concentrations of MgO, TiO2, K2O, Sr, Ba, and La of both samples are displayed in Figures 7 and 8, together with the composition of the basaltic to dacitic San Pedro lavas, and the calculated melts in equilibrium with hornblende (partition coefficients for dacitic melts of Sisson, 1994). Melts in equilibrium with plagioclase from both samples show the same compositional trends, although those displayed by sample Hx14b (Fig. 8) are somewhat more scattered than those of sample Hx14n (Fig. 7).
The trends displayed by the calculated melts in equilibrium with plagioclase show the same events that have been discussed up to now:
-
melts in equilibrium with plagioclase cores display increasing concentrations of TiO2, Sr, Ba, K2O, La, and Ce with decreasing MgO contents and mimic the trends displayed by the basaltic to basaltic andesitic lavas, which is consistent with the crystallization of olivine+clinopyroxene+anorthitic plagioclase (Figs. 7 and 8). The calculated TiO2 concentrations also increase with Sr concentrations, although it is not clear why the trends are different from those displayed by the basaltic to basaltic andesitic lavas.
-
melts in equilibrium with the core-rim transition zones display dramatic variations, comparable to the range of basaltic to andesitic lava whole-rock compositions.
-
melts in equilibrium with plagioclase rims have lower TiO2, FeO* (not shown) and Sr, and higher K, Ba, and La than melts in equilibrium with plagioclase cores. Such compositional differences can be explained primarily by the crystallization of hornblende (± orthopyroxene and phlogopite). The calculated melts in equilibrium with hornblende show TiO2, Sr, La, and Ce concentrations that range from those of the basaltic andesitic to dacitic lavas, and further support the hypothesis that hornblende crystallization began just prior to crystallization of the plagioclase rims. Ideally, one could use the calculated melts to perform quantitative modeling and determine if the abrupt compositional changes between cores and rims are due to closed system crystallization of hornblende+orthopyroxene +phlogopite, or if these minerals crystallized after the melt migration and reaction hypothesis discussed above. Unfortunately, the uncertainties in the partition coefficients preclude such an approach (see discussion below).
-
melts in equilibrium with plagioclase rims display decreasing Sr and Ba concentrations and increasing K/Ba and K2O concentrations, whereas TiO2, MgO, La, and Ce abundances remain more or less constant (Figs. 7 and 8). These trends are not displayed by the San Pedro lavas, suggesting that rims have recorded the evolution from dacitic to rhyolitic liquid compositions through crystallization of a mineral assemblage dominated by phlogopite and anorthite-poor plagioclase (e.g., <An40). The fact that La and Ce concentrations remain more or less constant could be explained by the crystallization of apatite (present in the glass) or by the high partition coefficients for La and Ce that have been reported for some biotites and may also apply to phlogopite (e.g., Mahood & Hildreth, 1983; Nash & Crecraft, 1985).
 Figure 7. Variation diagrams of calculated compositions of the melts in equilibrium with plagioclase of sample Hx14n.
Figure 7. Variation diagrams of calculated compositions of the melts in equilibrium with plagioclase of sample Hx14n.
Also shown are the calculated compositions of the melts in equilibrium with Hbl and the composition of the interstitial glass. The dashed line and arrow indicate the inferred differentiation trend of Volcán San Pedro lavas. The 2-s precision of the calculated melts is shown by horizontal and vertical bars for two points in each variation diagram. See text for discussion.
 Figure 8. Variation diagrams of calculated compositions of the melts in equilibrium with plagioclase of sample Hx14b.
Figure 8. Variation diagrams of calculated compositions of the melts in equilibrium with plagioclase of sample Hx14b.
Also shown are the calculated compositions of the melts in equilibrium with hornblende and the composition of the interstitial glass. The 2-s precision of the calculated melts is shown by horizontal and vertical bars for two points in each variation diagram. The dashed line and arrow indicate the inferred differentiation trend of Volcán San Pedro lavas. See text for discussion.
Although melts in equilibrium with plagioclase rims display trends in accord with a fractionation from dacitic to rhyolitic melt, the absolute calculated concentrations are unlike those of the interstitial rhyolitic glass and unlike those of natural magmas found at the TSPC. To further illustrate this (Fig. 9) we have compared the calculated compositions with the dacitic to rhyolitic compositions of TSPC lavas and of two Miocene granitoid plutons from the basement of the TSPC (Nelson et al., 1999). The calculated melts in equilibrium with plagioclase rims show similar differentiation trends to those of the plutons but the absolute calculated concentrations of Ba and K2O are shifted by about 50 % towards lower concentrations. The calculated La and Ce concentrations are shifted by a similar amount but towards higher values (not shown). Analytical problems with the ion microprobe trace element analyses of plagioclase can be discarded (Appendix I). Thus, the discrepancy between the calculated compositions and those of natural liquids at the TSPC has to be due to other factors. We will consider the following:
-
protracted closed-system crystallization may have led to individual pockets of melt that evolved independently. If this were the case, the composition recorded by every plagioclase crystal should be different and would reflect the composition of the local environment. The relatively minor differences between the major and trace element zoning profiles of plagioclase crystals Pl2n and Pl7n argues against the existence of pockets of melt that evolved in vastly different ways.
-
crystallization kinetics may result in partitioning relations quite different from the equilibrium case if the rate of crystal growth is much faster than chemical diffusion in the melt. Fast crystal growth results in apparent partition coefficients that are higher than equilibrium values for elements with D<<1, and lower than equilibrium values for elements with D>>1 (see equation 5 of Smith et al., 1955). The low La and Ce diffusivities in silicate melts (e.g., Brady, 1995) together with their small plagioclase-melt partition coefficients (DLa = 0.19 and DCe = 0.15, at 950oC and An40; Bindeman et al., 1998), would suggest that their high calculated concentrations can be explained at least in part by high plagioclase growth rates. If this were the case, similar effects should be observed in the behavior of K2O, as DK (0.13; at 950oC and An40; Bindeman et al., 1998) is smaller than DLa and DCe. As the calculated K2O concentrations are low when compared to dacitic and rhyolitic compositions, high plagioclase growth rates seems not to be the explanation for the anomalous calculated compositions.
-
the partition coefficients derived from the empirical equations of Blundy & Wood (1991) and Bindeman et al. (1998) might not be appropriate for the evolved melt and plagioclase compositions considered here. To evaluate this we have plotted the calculated liquid compositions in equilibrium with the plagioclase phenocrysts of the host dacite lava (Fig. 9). The calculated concentrations of Ba and Sr in equilibrium with the dacite plagioclase phenocryst are comparable to those of the bulk-rock dacites of San Pedro, whereas the calculated K2O concentration is ~ 50 % lower. This would suggest that DSr and DBa are appropriate for plagioclase-melt compositions studied here, whereas DK is not. The DK (0.13 ± 0.02) should be about 50 % smaller, and DLa (0.19 ± 0.08) and DCe (0.15 ± 0.04) should be about 50 % larger for the composition of the calculated melts in equilibrium with plagioclase rims to lie between those of the San Pedro dacite and the rhyolitic glass. Such partition coefficients have been reported in the literature, and it is apparent from the compilations of plagioclase-melt partition coefficients of Bindeman et al. (1998) that at ~ An40, estimates of DK vary from 0.08 to 0.24, DLa from 0.24 to 0.5, and DCe 0.22 to 0.3. Thus, the discrepancy between the calculated compositions in equilibrium with plagioclase rims and those of dacitic to rhyolitic natural liquids (including xenoliths interstitial glass) found in TSPC could be partly explained if the partition coefficients of Bindeman et al. (1998) were not suited for calculating plagioclase-melt partition coefficients in water-rich silicic systems.
-
As the elements that show low calculated concentrations are those that are highly compatible in phlogopite (K, Ba, and Rb), it seems plausible that during melt percolation through the crystal-network, phlogopite crystallization kept the K, Ba, and Rb concentrations low, and once phlogopite crystallization stopped (and melt migration continued) the liquids returned to more "usual" compositions. In this scenario, the composition of the interstitial glass would not be the product of protracted crystallization but would represent the composition comparable to that of the migrating liquid.
 Figure 9. Variation diagrams of calculated compositions of the melts in equilibrium with plagioclase of both samples.
Figure 9. Variation diagrams of calculated compositions of the melts in equilibrium with plagioclase of both samples.
The compositions of dacitic to rhyolitic lavas and plutons (triangles) of the TSPC display an inferred differentiation trend indicated by the dashed line and arrow. Squares are the calculated composition in equilibrium with plagioclase phenocrysts of the host dacite lava; the other symbols are the same as in Figs. 7 and 8. See text for discussion.
Conclusions
Textural, major and trace element zoning of plagioclase combined with the compositions of interstitial glasses of a suite of gabbroic xenoliths has provided a unique tool to trace the complex and protracted crystallization histories that may occur in partially crystallized zones of magma chambers. The compositions and trends displayed by the calculated melts in equilibrium with plagioclase indicate that after an early episode of differentiation from basaltic to basaltic andesitic liquids, the interstitial melt changed abruptly to dacitic compositions. This can be best explained by melt migration through a largely crystalline framework. The mafic interstitial liquid was displaced by a dacitic melt that reacted with the pre-existing cumulus minerals (olivine, clinopyroxene and Cr-spinel), and formed orthopyroxene, hornblende, and phlogopite. During and after this event, the interstitial melt evolved to rhyolitic compositions. However, minor and trace element compositions in liquids calculated from evolved plagioclase (e.g., < An40) are unlike those in the interstitial rhyolitic glass of the xenoliths or unlike dacitic to rhyolitic magmas found at the TSPC. This could be due to the complex nature of the melt migration and reaction processes that may occur in partially crystallized zones of magma chambers, or to uncertainties in the plagioclase-melt partition coefficients that apply to water-rich silicic magmas.
Acknowledgements
I acknowledge R. Hinton for his collaboration, patience and hospitality during the ion microprobe analyses. J. -M. Boccard assisted with the painful drilling of the thin sections for ion microprobe analyses. I also thank S. Chakraborty for the discussions about Mg diffusion in plagioclase.
Appendix
Appendix I. Analytical methods
Electron microprobe analyses were done on a Cameca SX50 at the University of Lausanne, with wavelength dispersive spectrometry. Hornblende, plagioclase and glass were analyzed with an accelerating voltage of 15kV and beam currents of 15nA for hornblende and plagioclase, and 7 nA for glass. Beam diameter was ~ 2µm for plagioclase and hornblende, whereas for glass large areas were rastered when possible (~ 25µm2).
Ion-microprobe analyses were performed at the University of Edinburgh using a Cameca IMS-4f ion microprobe with a O- primary beam of net impact energy of 15 keV (10.7 keV primary and 4.5 keV secondary) and an operating current of 5-8 nA. The secondary ion accelerating voltage was 4500 keV. Only ions with energies falling between 55 and 95 eV (75+/-20 eV) were analyzed to reduce molecular ion interferences. Beam diameter ranged between 15 to 30 mm. For plagioclase and glass, the following isotopes were measured: 7Li, 26Mg, 30Si, 41K, 42Ca, 47Ti, 56Fe, 85Rb, 88Sr, 89Y, 138Ba, 139La, 140Ce, plus mass 60 for CaO overlap on Fe, and mass 130.5 to monitor the background. For hornblende the following isotopes were measured: 7Li, 30Si, 41K, 47Ti, 85Rb, 88Sr, 89Y, 93Nb, 138Ba, 139La, 140Ce, 141Pr, 143Nd, 149Sm, 151Eu, 156Gd, 157Gd, 159Tb, 161Dy, 165Ho, 167Er, 172Yb, plus mass 130.5 to monitor the background, and mass 154 for BaO overlap on Eu. Mass 156 is a combination of CeO and minor 156Gd and was used to calculate the overlap of the light REE on the heavy REE by assuming that REEO/REE follows CeO/Ce. The overlap of Si2 on Fe could not be corrected directly but was calculated using Fe-free systems to be equal to 229 ppm on all feldspars (R. Hinton, personal communication). The concentrations were obtained by normalization of mass 30Si to the silica values obtained by electron microprobe analyses. The SRM-610 glass standard was used as a monitor of day to day changes instrumental conditions (the nominal concentrations for SRM 610 are given in Hinton, 1990). The ion yields for glass and plagioclase relative to SRM-610 were determined using Lake County (LC) plagioclase, an alkali feldspar SHF1 (AF), and Corning glass (CG). Corning glass has similar ion yields to feldspars for elements such as K, Sr, and Ba and is assumed to give similar ion yields for those elements not available in the feldspar standards. The correction factors are as follows: 0.921 for Li (LC), 0.881 for Mg (LC), 0.768 for K (LC), 0.788 for Ca (LC), 0.793 for Ti (LC), 0.76 for Fe (CG), 0.768 for Rb (LC), 0.796 for Sr (LC), 0.796 for Y (LC), 0.773 for Ba (LC), 0.858 for La and Ce (AF). No ion-yield corrections were done for hornblende, because the values obtained using SRM 610 give good agreement with bulk analyses (R. Hinton, personal communication). The 2-s precision for most elements is under 5 % relative. Only La and Ce are in some cases slightly above 10 %, and Y and Rb are around 25 %.
Comparison of the electron and ion-microprobe concentrations of MgO, K2O and BaO agree within 10%, whereas FeO* determined by the electron-microprobe is ~ 20% higher than determined with the ion-microprobe. Sanidine analyses from O. Bachmann. (see the following figure).

Appendix II. Error of the plagioclase-melt partition coefficients used
The error associated with the partition coefficients determined by using equations of Blundy & Wood (1991) and Bindeman et al. (1998) was derived through error propagation (assuming independent errors; Taylor, 1982) of the uncertainties in their regressed parameters only. We did not take into account the error in An mol % determination from the electron microprobe analyses, the error in the trace element composition of plagioclase from ion-microprobe analyses, or the error in the temperature estimations from geothermometers. The derived equation is:
|D| = {{[[XAn exp((a XAn + b )/ RT)]/RT] |a|}^2 +
{[[exp((a XAn + b )/ RT)]/ RT] |b|}^2}^0.5
Where |D| is the absolute error on the partition coefficient, |a| and |b| are the absolute errors on the regressed values of a and b, and the rest of the symbols are the same as for equation (1).
IV. Magmatic sodium-rich phlogopite in suite of gabbroic crustal xenoliths from volcan San Pedro(36°S, Chilean Andes): evidence for a solvus relation between sodium phlogopite and phlogopite
Abstract
We present a textural and compositional study of the first occurrence of magmatic Na-rich phlogopite (1 to 5 wt % Na2O) in a calc-alkaline (medium-K) magmatic system. Phlogopite occurs as a late-crystallizing mineral in two groups of gabbroic crustal xenoliths at Volcán San Pedro (36o S, Chilean Andes), in reaction relation with olivine, pyroxenes, Cr-spinel, and in some cases plagioclase. The high mg-number, high Cr2O3 contents and moderate Na2O contents (e.g., 2 wt %) of some phlogopites can be explained by open-system processes involving migration of water-rich evolved melts and reaction with the pre-existing mafic minerals. However, the extremely high Na/K (up to 2.2) and Na2O concentrations (up to 5 wt %) of other phlogopites seem to require reaction with liquids of unrealistically high Na/K. Electron microprobe analyses reveal that these phlogopites are not homogeneous but consist of fine intergrowths (< 2 µm) of Na-rich and Na-poor phlogopite. This is best interpreted in terms of a solvus between sodic and potassic phlogopite end-members. The presence of Na-rich phlogopite in two groups of gabbroic xenoliths with different ages suggest that it might be a more common mineral than has been recognized previously in calc-alkaline systems.
1. Introduction
Magmatic Na-rich phlogopite (e.g., Na2O >1wt %) is an unusual mineral. Up to now, it has been exclusively recognized as minute mono- or polymineralic inclusions in chromites of layered intrusions (Muskox, Irvine, 1975; Bushveld Complex, Morette et al., 1984; Stillwater complex, Talkington et al., 1986; Laouni intrusion, Lorand & Cottin, 1987) and ophiolite complexes (Hongguleleng ophiolite, Peng et al., 1995; mid-oceanic ridge mafic and ultramafic rocks, Arai et al., 1997; Oman ophiolite, Schiano et al., 1997). Na-rich phlogopite is commonly present in association with phlogopite, olivine, pyroxene, albite, feldspathoids, and Na-rich amphibole (Table 1). The unusual occurrence of sodium phlogopite (or aspidolite; Rieder et al., 1998) in these systems has been interpreted as the result of mixing between primitive water-poor magmas and aqueous liquids, or as the product of reactions between anhydrous minerals (olivine, pyroxenes, and plagioclase) and aqueous liquids.
We document the first occurrence of Na-rich phlogopite (1 to 5 wt % Na2O) in two groups of crustal gabbroic xenoliths of the calc-alkaline Volcán San Pedro (36o S, Chilean Andes). We propose that open-system processes involving migration of evolved water-rich melts and reaction with early-crystallized mafic minerals (olivine, Cr-spinel, and pyroxenes) can explain the occurrence of phlogopites with moderate sodium contents (~ 2 wt %) in the San Pedro xenoliths. The highest Na2O concentrations (up to 5 wt %) and Na/K (up to 2.2) of other phlogopites could be explained if there is a solvus between the sodic and potassic phlogopite end-members, analogous to the solvus reported for the sodium (paragonite) and potassium (muscovite) white micas (e.g., Guidotti et al., 1994).
| Table 1. Occurrences of magmatic Na-rich phlogopite. |
| Reference |
Magmatic system |
Rock type |
Textural observations |
Na2O wt% in phlogopite |
| Irvine (1975) |
Muskox intrusion |
chromitite |
inclusion in Cr-spinel |
not reported |
| Morette et al. (1984) |
Critical Zone of the Bushveld complex |
chromitite |
inclusion in Cr-spinel |
6.4 |
| Talkington et al. (1986) |
J-M reef, Stillwater complex |
chromitite |
inclusion in Cr-spinel |
< ;1-7.1 |
| Lorand & Cottin (1987) |
Laouni intrusion (Algeria) |
olivine-gabbro |
inclusion in Cr-spinel |
< ;1-7.2 |
| Peng et al. (1995) |
Hongguleleng ophiolite (China) |
plagioclase dunite and chromitite |
inclusion in Cr-spinel |
< ;1-6.3 |
| Arai et al. (1997) |
MOR ultramafic and mafic plutonic rocks |
gabbros and troctolites |
inclusion in Cr-spinel |
< ;1-5.5 |
| Schiano et al. (1997) |
Oman ophiolite |
chromitite |
inclusion in Cr-spinel |
3.2-6.8 |
| This work |
Calc-alkaline Volcán San Pedro xenoliths |
olivine-horblende norites |
poikilitic. Surrounding resorbed Ol and Cr-spinel |
1-3.5 |
| This work |
Calc-alkaline Volcán San Pedro xenoliths |
Hornblende norites |
poikilitic. Surrounding resorbed Ol, Opx, Cr-spinel, and Pl |
1-5 |
| This work |
Calc-alkaline Volcán San Pedro xenoliths |
Clinopyroxene leuconorites |
poikilitic and in microfractures |
1-2.1 |
2. Geological setting
Holocene Volcán San Pedro is the youngest and most prominent volcanic edifice (3621 m) of the Quaternary Tatara-San Pedro Complex (~ 55 km3; TSPC), which is located on the volcanic front of the Southern Volcanic Zone (SVZ) of the Andes, at 36o S, 71o51'W (Singer et al., 1997; see Figs. 1 and 2 of Chapter II). The TSPC comprises roughly 1 Ma of volcanic activity, consisting mainly of lavas ranging from basalt to rhyolite that define medium to high-K calc-alkaline trends (Fig. 6 of Singer et al., 1997). The magmatic activity at Volcán San Pedro is divided into a cone-building phase comprising andesitic and dacitic lavas, and a younger phase that post-dates the sector collapse of the eastern flank of the volcano, which was accompanied by an explosive eruption that produced dacitic air-fall deposits (Singer & Dungan, 1992). This was followed by the eruption of a sequence of lava flows that apparently records the downward tapping of a strongly zoned magma chamber. The eruptive sequence includes: (1) 0.2 km3 of biotite-hornblende dacite containing abundant gabbroic xenoliths (up to 45 cm in diameter) and quenched mafic inclusions (QMI), (2) 0.5 km3 of two-pyroxene dacite with abundant QMI, and (3) 0.1 km3 of two-pyroxene andesite with rare QMI. The last volcanic activity consisted of 0.2 km3 of basaltic andesitic magma (55-57 wt % SiO2) which rebuilt the summit cone. The fact that the xenoliths have been found exclusively in the first lava following sector collapse and the ensuing explosive eruption suggests that they are fragments of the conduits or upper parts of the margin of the San Pedro magma chamber which were shattered and incorporated during the eruption (in a similar fashion to the May 18, 1980 Mount St. Helens eruption; Heliker, 1995).
3. Textures of the Gabbroic Xenoliths
The majority of the samples are clinopyroxene hornblende leuconorites and norites, with minor hornblende norites, and olivine norites (classification following Streckeisen, 1976; Le Maitre, 1989). All samples appear to be free of alteration or secondary hydrothermal minerals. On the basis of textural observations and modal abundances, we have divided the xenoliths into two main groups:
-
Group I consists of partially crystallized olivine-hornblende norites and melanorites. These are characterized by the presence of interstitial residual glass and thus are potentially co-magmatic with San Pedro magmas.
-
Group II consists of xenoliths with subsolidus and deformation textures. These have been further subdivided into: a) Group IICL are mainly clinopyroxene leuconorites with high proportions of plagioclase (> 50 vol. %), low olivine (< 10 vol. %) and variable amounts of hornblende (< 40 vol. %), and b) Group IIHN are hornblende norites with high modal proportions of poikilitic hornblende (> 30 vol. %), low plagioclase (< 35 vol. %) and significant amounts of olivine (> 10 vol. %). 40Ar/39Ar analyses suggest that these xenoliths are more than 1 Ma old (Chapter II), and thus they are fragments of the pre-Quaternary plutonic basement of the volcano.
 Figure 1. A. Ol-Hbl norite. Na-rich phlogopite surrrounding resorbed olivine (Ol).
Figure 1. A. Ol-Hbl norite. Na-rich phlogopite surrrounding resorbed olivine (Ol).
Note euhedral margins of the mica in contact with vesicular interstitial glass. B. Ol-Hbl norite. Two Na-rich phlogopite crystals surrounding resorbed Ol. C. Hbl-norite. Phlogopite crystal with up to 5 wt % Na2O; note resorbed plagioclase (Pl) and Ol in the vicinity. Black square is the area of X-ray map shown in Figure 3. D. Hbl-norite. Na-rich phlogopite surrounding corroded Pl. E. Cpx-leuconorite. Phlogopite surrounding corroded orthopyroxene (Opx) and Pl. F. Cpx-leuconorite. Microfracture filled with phlogopite.
3.1. Group I. Olivine-hornblende norites.
The samples consist of Cr-spinel, olivine, minor clinopyroxene, orthopyroxene, hornblende, plagioclase, and phlogopite forming a medium grained (1-5 mm) crystal network with interstitial vesiculated SiO2-rich (67-72 wt %) glass filling the interstices (0.5 13 vol. %). Glass is distributed in isolated and occasionally interconnected pockets bounded by euhedral crystal faces suggesting that it is residual from crystallization and not due to partial melting. Phlogopite makes up to 8 vol. % and is euhedral to subhedral. Euhedral phlogopite occurs in the interstitial glass attesting to a magmatic origin and indicating that it was among the last minerals to crystallize (Fig. 1). Phlogopite is almost invariably present surrounding resorbed olivine and Cr-spinel (Fig. 1), indicating that a reaction between these minerals and an evolved water-rich liquid occurred.
3.2. Group II. Hornblende norites and clinopyroxene leuconorites with subsolidus textures.
Clinopyroxene leuconorites display seriate, mosaic textures with abundant plagioclase (up to 80 vol. %), olivine, minor Cr-spinel, clinopyroxene, hornblende, Fe-Ti oxides and phlogopite. Exsolution lamellae in pyroxenes, bent twins in plagioclase, and the presence of microfractures record subsolidus cooling and deformation. Phlogopite (up to 15 vol. %) occurs as small (< 1 mm) anhedral crystals surrounding resorbed plagioclase, orthopyroxene, and Fe-Ti oxides (Fig. 1). Phlogopite also occurs filling discontinuous microfractures (< 0.5 mm in width) together with hornblende, orthopyroxene, and Fe-Ti oxides (Fig. 1).
Hornblende norites consist of large anhedral oikocrysts (> 1 cm across) of hornblende that enclose resorbed, anhedral olivine, Cr-spinel, clinopyroxene, orthopyroxene, plagioclase. Phlogopite makes up to 4 vol. % and is commonly subhedral to anhedral. It occurs inside hornblende crystals, surrounding resorbed olivine, Cr-spinel, orthopyroxene, and in contrast with the Group I xenoliths, it is also present surrounding resorbed plagioclase (Fig. 1).
4. Phlogopite compositions
Electron microprobe analyses (Cameca SX-50, University of Lausanne) of phlogopite and biotite of the host dacite were performed primarily as point analyses, but in some cases as core to rim traverses (Table 2). Structural formulae were determined following the normalization scheme of Dymek (1983, scheme C) which is based on 7 cations and 22 negative charges. The distinction between phlogopite and biotite follows Deer et al. (1962), where phlogopite has a ratio of magnesium to total iron per formula unit greater than two. The analytical details of the electron microprobe analyses are reported in the Appendix.
4.1. Group I xenoliths. Olivine-hornblende norites.
Phlogopites have mg-numbers (mg-number = 100*Mg/(Mg+Fet) in mols; Fet= total iron) ranging from 82 to 77, and Cr2O3 concentrations from <0.05 to 0.43 wt %. The Na2O contents are between 1.5 and 3.5 wt %, and Na/K apfu (atoms per formula unit) values vary between 0.17 and 0.94 (Fig.2). The occupancy of the A-site ranges 0.9 to 1.0, which differentiates these phlogopites from the interlayer-deficient mica wonesite (Spear et al., 1981). The major element composition (e.g., MgO) of the phlogopites is comparable among different crystals, but minor elements (particularly Cr and Ba) are highly variable (Fig. 2). We interpret the presence of phlogopite with high Cr2O3 contents and mg-numbers as due to reactions between olivine + Cr-spinel and an evolved water-bearing liquid. Volfinger et al. (1985) suggested that high Mg/Fet in mica enhances substitution of Na for K. The composition of phlogopites in San Pedro xenoliths show a broadly positive correlation between the mg-number and the Na/K values (Fig. 2), and thus it seems plausible that high Na/K of the phlogopites is partly due to their high mg-numbers.
 Figure 2. Variation diagrams of the phlogopite compositions from the xenoliths and of the biotites from the host dacite lava.
Figure 2. Variation diagrams of the phlogopite compositions from the xenoliths and of the biotites from the host dacite lava.
4.2. Group IICL xenoliths. Clinopyroxene leuconorites.
Poikilitic phlogopites have mg-numbers that vary from 81-70 and Cr2O3 contents are typically < 0.2 wt %. Their Na2O contents (1.1-2.1 wt %) and Na/K (0.2-0.4) are lower than those of phlogopites from Group I xenoliths (Fig. 2). Phlogopites filling microfractures have lower mg-numbers (76-70), lower Cr2O3 (< 0.15 wt %) and Na2O (0.7-1.3 wt%) contents, and lower Na/K (~ 0.2) than poikilitic phlogopites. The structural formulae (Table 2) shows that some poikilitic phlogopite crystals have the tetrahedral position partly filled with Fe3+, whereas phlogopites filling microfractures and biotites of the dacite host lava have invariably Fe3+ in the tetrahedral position. Oxygen fugacity estimates of the host dacite using ilmenite and magnetite pairs are close to NNO+2 (B.S. Singer, unpublished data) which is in support with the observation of Briggati et al. (1996) that Fe3+ in the tetrahedral position reflects crystallization at low pressure and high fO2 conditions. We propose that phlogopites filling microfractures crystallized from oxidized, evolved water-rich liquids comparable to the host dacite. The higher mg-number and Cr2O3 contents of the poikilitic phlogopites compared to those filling microfractures are interpreted to be the result of reactions between mafic minerals (e.g., olivine, Cr-spinel, and pyroxenes) and evolved melts that migrated through the microfractures.
 Figure 3. X-ray map of Na and K distribution of the phlogopite with up to 5 wt % Na2O.
Figure 3. X-ray map of Na and K distribution of the phlogopite with up to 5 wt % Na2O.
Na and K are distributed as bands approximately parallel to the cleavage. The mapped area is marked in Figure 1c. The dashed line marks the position of the electron microprobe traverse shown in Figure 4.
4.3. Group IIHN xenoliths. Hornblende norites.
Phlogopites have mg-numbers ranging from 84-77 and Cr2O3 contents up to 0.2 wt %. Most phlogopites have Na2O contents between 1 and 2.5 wt %, except for one poikilitic crystal (3 mm across; Fig. 1c) that has up to 5 wt % Na2O, and Na/K mol values up to 2.2 (Fig. 2). An X-ray map of this phlogopite crystal (Fig. 3) shows that the sodium concentration is not homogeneous. The crystal consists of Na-rich bands (< 50 µm) that are parallel to the phlogopite cleavage (approximately parallel to the 001 plane) and which alternate with K-poor zones. An electron microprobe traverse across the Na-rich zone of this phlogopite crystal (Fig. 4) shows that variations of up to 2 wt % of Na2O occur in less than 10 µm, suggesting that even the Na-rich zones are heterogeneous and probably consists of fine (< 1-2 µm) intergrowths of Na-rich phlogopite and phlogopite. Such abrupt changes in the phlogopite Na2O and K2O concentrations are difficult to explain by changes in the liquid composition alone, and they probably reflect the presence of a solvus.
 Figure 4. Traverse across a Na-rich zone of the phlogopite crystal shown in Figure 3.
Figure 4. Traverse across a Na-rich zone of the phlogopite crystal shown in Figure 3.
The Na-rich zone is complementary with K-poor zones. Within the Na-rich zone variations of up to 2 wt % of Na2O occurs, suggesting that it consists of mixtures of almost pure sodium phlogopite and phlogopite. Note that the spacing of the electron microprobe analyses in the Na-rich zone is 5 times smaller (2 µm) than for the rest of the traverse.
5. Discussion
5.1. Genesis of the Na-rich phlogopites
The presence and composition (e.g., high Cr2O3 and mg-number) of the phlogopites in the San Pedro gabbroic xenoliths can be explained by reactions between evolved water-rich liquids and olivine, pyroxenes, and Cr-spinel. We propose that these reactions were not due to closed-system crystallization because experiments on medium-K calc-alkaline water-rich liquids (e.g., Sisson & Grove, 1993) have not reported Na-rich phlogopite, or phlogopite even at crystallinities of up to 90 % (Kawamoto, 1996). Moreover, the microfractures filled with phlogopite in Group II xenoliths are evidence of open-system processes involving migration of evolved water-rich melts (see also Chapter II).
To explain the high Na/K values of most phlogopites we consider below: (1) the possibility that plagioclase was involved in the phlogopite-forming reactions, (2) the composition of the reacting liquids, and (3) the potential role of a solvus.
5.1.1. Is plagioclase responsible for the high Na/K of the phlogopites?
Textural relations of Group II xenoliths suggest that plagioclase could have participated in the phlogopite-forming reaction, and thus it could be responsible at least in part for the high Na/K of phlogopite. However, this does not explain the high Na/K of phlogopites in Group I xenoliths, where textural relations indicate that plagioclase was stable during phlogopite crystallization. The fact sodium is concentrated in bands instead of being homogeneously distributed in the phlogopite crystal with the highest Na2O content (Fig. 3) also argues against plagioclase being entirely responsible for the high Na/K of the phlogopites. The apparent reaction relation between phlogopite and plagioclase in Group II xenoliths could be explained if we envisage a scenario where plagioclase dissolved into the reacting liquid, and later phlogopite passively crystallized around resorbed plagioclase.
5.1.2. Composition of the reacting liquids
The composition of the liquid from which the San Pedro phlogopites crystallized can be assessed by comparing their Na/K values to those of phlogopites crystallized from experiments. Aside from K-free experiments of Carman (1974), the only other experiments that have produced phlogopite with significant amount of Na2O (e.g., > 1 wt %) are those of Barclay & Carmichael (in prep.) on a trachybasaltic composition, and those of Prouteau & Scaillet (personal communication; Prouteau, 1999) produced by the interaction between trondhjemitic liquids and forsteritic olivine. Figure 5 shows the lines joining phlogopite and coexisting liquid composition from experiments and those of biotite and interstitial glass of the host dacite lava. We propose that phlogopites of the San Pedro xenoliths with Na# values (Na# = 100*Na/(Na+K) in mols) of up to ~ 15, which corresponds to ~ 2 wt % Na2O, could have crystallized from liquid compositions comparable to the dacites of Volcán San Pedro (Fig. 5).
The rest of the phlogopites have Na# (up to 55) that seem to require reaction with liquids of unrealistically high Na#. Even the phlogopite that crystallized from trondhjemitic liquids have Na# values of only 27 (Fig. 5).
 Figure 5. Phologopite-coexisting liquid tie lines from experiments of Barclay & Carmichael (B&C, in prep) and Prouteau & Scaillet (P & S; personal communication).
Figure 5. Phologopite-coexisting liquid tie lines from experiments of Barclay & Carmichael (B&C, in prep) and Prouteau & Scaillet (P & S; personal communication).
Also plotted are the tie lines between biotite and interstitial glass of the host dacite. The compositions of phlogopites from ophiolite complexes are from: Hongguleleng ophiolite (Peng et al., 1995); mid-oceanic ridge mafic and ultramafic rocks (Arai et al., 1997); Oman ophiolite (Schiano et al., 1997). Data from the Laouni intrusion (Lorand & Cottin, 1987) is also included. See text for discussion.
5.1.3. A solvus relation among phlogopite and sodium phlogopite?
The extremely high Na# (55) and Na2O contents (5 wt %) of some phlogopites could be explained if there were a solvus between the sodium and potassium phlogopite end-members. Supporting evidence for the existence of such a solvus are the mode of occurrence and composition of phlogopites present in chromite inclusions of ophiolite complexes.
In many examples, almost pure sodium and potassium phlogopite coexist as separate crystals inside a single chromite grain (e.g., Peng et al., 1995; Schiano et al., 1997) suggesting that both phlogopites co-crystallized from a single liquid. In the San Pedro xenoliths, there are no discrete phlogopite crystals with end-member compositions, instead the phlogopite with the highest sodium contents consists of fine intergrowths (< 2 µm) of Na-rich and Na-poor phlogopite (Figs. 3 and 4). As these intergrowths are about the size of the electron microprobe beam, the analyzed phlogopites have compositions that reflect mixtures between Na-rich and Na-poor phlogopite, as has been found in other studies of submicroscopically intergrowths of micas (e.g., Shau et al., 1991). The proposed solvus between the Na and K phlogopite end-members is probably analogous to that recognized for the sodium (paragonite) and potassium (muscovite) white micas (e.g., Guidotti et al., 1994).
Appendix. Analytical techniques
Electron microprobe analyses where performed with a SX-50 Cameca instrument at the University of Lausanne. Accelerating voltage was 15 kV, beam current 15 nA, and beam diameter of 2 µm. Typical 2-s relative precisions (in %) are as follows: SiO2 (0.9), TiO2 (5-10), Al2O3 (1-1.5), Cr2O3 (12-40), FeO* (3), MnO (30 to below detection), MgO (1), CaO (30 to below detection), Na2O (3-6), K2O (2-2.5), BaO (25 to below detection), F (25 to below detection), Cl (30-50). X-ray map of Na and K was done by rastering the electron microprobe beam with an accelerating voltage of 15 kV and beam current of 30nA.











































