2 Introduction
Periodontal diseases are considered as infectious processes with bacterial etiology (Socransky 1977, Socransky & Haffajee 1994). Thus the adjunctive use of antimicrobial agents, either systemically or locally, has been advocated in order to enhance the effect of classical mechanical therapy (Listgarten & Hellden 1978, Goodson et al. 1979, Loesche et al. 1981). In subjects with periodontitis, all parts of the dentition are usually not affected with equal severity. It has therefore been suggested to focus therapy on the affected areas and to limit the delivery of drugs to the periodontal pockets. Local drug delivery systems have been developed aiming at longer duration and higher concentration of active agents directly at the site of action. For more details on local delivery systems, see section 2.1.
Recently developed biodegradable polymers, poly(ortho esters), have a number of interesting characteristics, such as biocompatibility, bioerodibility and injectability, which make them promising candidates as carriers of antimicrobial agents for local subgingival therapy. Some types of these polymers also have adhesive properties that may improve their retention on dental surfaces (Schwach-Abdellaoui et al. 2001). For more details on poly(ortho esters), see 2.2.
Tetracyclines comprise a group of antimicrobial agents widely used in the field of periodontology. Their bacteriostatic activity against a large panel of periodontal pathogens (Walker 1996), as well as their ability to inhibit collagenase (Golub et al. 1991) and to bind to the tooth surface (Baker et al. 1983), have made them popular for several decades. Tetracycline hydrochloride (TH), doxycycline and minocycline, all semi-synthetic tetracyclines, have been studied and used for the systemic route as well as for local applications (for review, see Seymour & Heasman 1995). Tetracycline base, having the same actions and uses as TH can easily be incorporated into poly(ortho ester) by mixing the two components. In vitro, the drug is released according to a zero order process, confirming that delivery occurs by surface erosion of the polymer (Schwach-Abdellaoui et al. 1998, Schwach-Abdellaoui et al. 2001). Previous in vitro results are presented in section 2.3.
2.1 Local Delivery Devices
As irrigation of pockets can achieve therapeutic drug levels for only short periods of time (Pitcher et al. 1980, Eakle et al. 1986, Oosterwaal et al. 1990), controlled delivery systems have been proposed to extend the time of action of locally applied antimicrobial agents. The use of such systems has been advocated particularly in the therapy of sites difficult to reach by mechanical means, for example furcation defects (Nordland et al. 1987), and sites not responding to conventional mechanical therapy (see Greenstein & Polson 1998 for review). Although numerous local controlled delivery systems have been designed in the past two decades, only few have been tested extensively, and even fewer have become commercially available for broad use by dentists (for review see Mombelli & Tonetti 2001).
Two multi-center controlled clinical trials have shown the efficacy a 2% minocycline ointment (Dentomycin, Lederle, Wayne, USA) to improve clinical parameters, if applied repeatedly. In the first study, subjects were treated 4 times in addition to scaling and root planing with minocycline or placebo ointment (van Steenberghe et al. 1993). The second study evaluated a seven-time repeated application of the drug over a 15-months period (van Steenberghe et al. 1993). The repeated subgingival administration of minocycline ointment yielded an adjunctive improvement after subgingival instrumentation in both clinical and microbiologic variables.
Another product contains metronidazole as active principle (25%) in glyceryl-mono-oleate and sesame oil as carriers (Elyzol®, Dumex, Copenhagen, Denmark). This degradable compound can be injected in the form of a gel with a syringe directly into the pocket. Although the gel increases its viscosity in the pocket due to the presence of water, about half of the product is lost immediately following placement, and in the hours thereafter the concentration of metronidazole decreases exponentially (Stoltze 1992, Stoltze 1995). The manufacturer therefore recommends two applications of the gel within one week. In clinical studies no significant difference in periodontal parameters was demonstrated between Elyzol® and conventional SRP (Ainamo et al. 1992, Grossi et al. 1995, Pihlstrom et al. 1995); these results were interpreted to suggest equivalence of the two treatments.
In one of the first clinical trials testing the controlled local administration of a drug into a periodontal pocket, delivery of tetracycline was attempted by placing hollow dialysis tubes filled with the active agent into the diseased sites (Goodson et al. 1979). Subsequent developments resulted in the Actisite® tetracycline fiber (Alza, Palo Alto, USA), consisting of a monolithic ethylene-vinyl-acetate copolymer loaded with tetracycline hydrochloride crystals (Goodson et al. 1979). The nondegradable carrier assumes the form of a fiber with a diameter of 0.5 mm and a length of 23 cm, and contains 12.7 mg of the antimicrobial agent. The device is densely packed into the periodontal pocket and left in situ during 7 to 10 days. Measurements in humans have shown very high and constant concentrations of drug at the treated site during the entire time of application (Tonetti et al. 1990). In several multi-center studies, the tetracycline fiber has proved to be effective. Used as an adjunct to scaling and root planing (SRP), it improved probing pocket depth (PPD) and clinical attachment level (CAL) significantly better than mechanical treatment alone (Newman et al. 1994, Michalowicz et al. 1995). Despite of its proven efficacy, the device has been withdrawn from the market in early 2002, and is presently no longer available commercially.
A more recently developed system contains 8.5% doxycycline hyclate as active agent. Polylactic acid is the biodegradable vehicle and N-methyl-2-pyrrolidone (NMP) the solvent (Atridox, Atrix Laboratories, Ft.Collins, USA). Two separate syringes are coupled and mixed just prior to use. As soon as the compound is injected into the pocket, the NMP is replaced by water, which causes the polymer to return to its solid state (Stoller et al. 1998). On the short term, clinical studies have demonstrated that chemical therapy alone is at least as effective as SRP in improving clinical parameters (Garrett et al. 1999, Garrett et al. 2000). In one study, even better results were obtained in a group treated with the drug during a single session of scaling, compared to those obtained in subjects treated in four appointments with subgingival mechanical therapy (Wennstrom et al 2001).
The potential usefulness of microcapsules as local delivery device for periodontal therapy has also been investigated. In 1994, (Jones et al.) described the use of minocycline HCl microencapsulated in poly(lactic acid-co-glycolic acid) (PLGA) for the treatment of chronic periodontitis. The biodegradable polymer containing the active agent had the form of a powder and was administered into the periodontal pocket with a syringe. Maybe due to the small number of subjects and/or insufficient plaque control, clinical and microbiological parameters failed to demonstrate superiority in comparison to control or mechanical treatment alone (Jones et al. 1994). A few years later, Yeom (1997) presented a new bioabsorbable polymeric system consisting of chitosan-encapsulated alginate microspheres loaded with 10% minocycline. The injectable polymeric compound was tested in 15 patients as adjunct to supragingival scaling compared to SRP alone. The six-week observation period permitted to conclude that BoP was better reduced by antimicrobial therapy than by SRP. Microbiological indicators of periodontal health tended to be better in minocycline sites than SRP sites (Yeom et al. 1997). A multicentric study, including nearly 750 subjects in 18 centers, demonstrated the superiority of locally delivered minocycline (in PLGA) over nine months upon SRP alone, or SRP plus vehicle (Williams et al. 2001).
2.2 Poly (ortho esters)
Because their physico-chemical properties can be varied to a large extent, polymers attract much attention for their potential use as biomaterials in various fields of medicine. A wide range of applications are currently explored in orthopedics, ophthalmology, or dentistry, including their potential use as carriers of active agents for controlled local drug delivery (Sintzel et al. 1996, Peter et al. 1998).
Poly(ortho esters) (POE) are synthetic biodegradable hydrophobic polymers that allow the liberation of incorporated agents by surface erosion. This mechanism has been validated in animal models for applications in ophthalmology (Einmahl et al. 2000, Einmahl et al. 2001a, Merkli et al. 1994, Zignani et al. 1998, Zignani et al. 2000b). The use of semi-solid POEs is expected to lead to significant advances in glaucoma filtering surgery, and may make drug delivery to various parts of the human eye possible (Zignani et al. 2000a, Einmahl et al. 2002). Investigations are currently in progress in several other fields of veterinary and human medicine (for review see Einmahl et al. 2001b). POEs may also become suitable candidates for local controlled drug delivery in periodontal pockets. Preliminary laboratory investigations have been presented (Roskos et al. 1995.
The polymer described in the present work is a low molecular weight auto-catalyzed POE containing 30 mol% of lactic acid units (POE70LA30). It was recently synthesized, characterized and optimized (Schwach-Abdellaoui et al. 1999, Schwach-Abdellaoui et al. 2001). Synthesis reaction is an acid-catalyzed condensation of 3,9-diethylidene-2,4,8,10-tetraoxaspiro[5,5]undecane (DETOSU) with 1,10decanediol and 1,10decanediol-dilactate. ptoluenesufonic acid (p-TSA) is added as catalyst and tetrahydrofuran (THF) is the solvent (Figure 1).

By modifying the proportion of lactic acid in the polymer, different degradation rates can be obtained (see 2.3) and the physicochemical properties of the compound (POExLAy) can be varied. For example, POE70LA30 shows a low viscosity and, therefore, is suitable for injectable preparations (Schwach-Abdellaoui et al. 2001).
2.3 Previous experiments
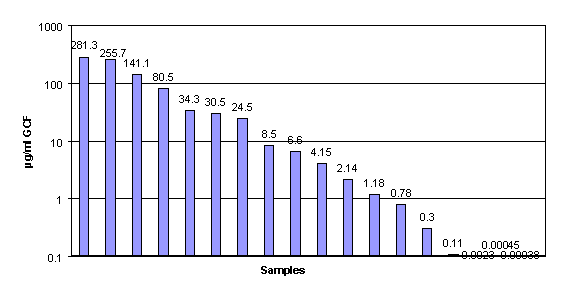
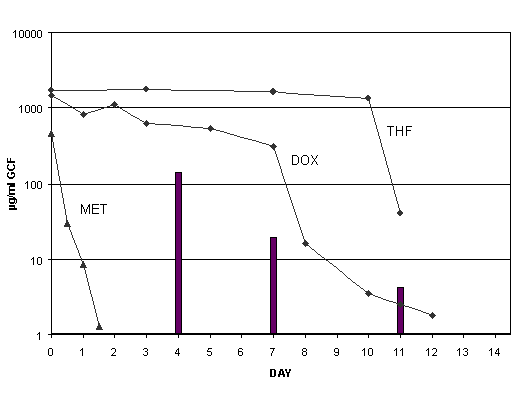
Degradation of auto-catalyzed POE occurs mostly by surface erosion. An incorporated drug could therefore be released for days to weeks (Ng et al. 1997, Schwach-Abdellaoui et al. 1998, Sintzel et al. 1998a, Sintzel et al. 1998b, Schwach-Abdellaoui et al. 1999). Delivery of tetracycline incorporated in POE70LA30 has been previously studied in vitro in thermostated cells at 37°C, circulated with phosphate buffer (Schwach-Abdellaoui et al. 2001). The released amount of drug was measured by high-performance liquid chromatography (HPLC). Figure 2 shows, for 10% or 20% tetracycline load, that the delivery lasted two weeks at an almost constant rate. The complete degradation of the polymeric matrix was simultaneous.

2.4 Purpose
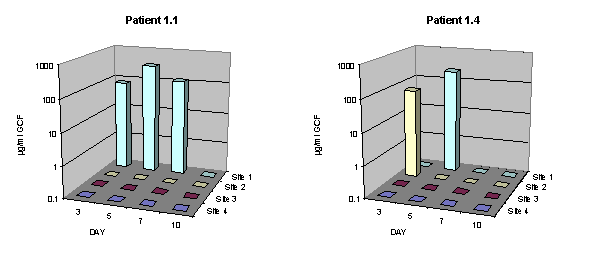
The aim of this phase I in vivo study was to explore the clinical potential of poly(ortho esters) as carriers of antimicrobial agents for the local treatment of periodontal disease. Observations focused on the retention of the material in periodontal pockets and the tolerance to the product. Additionally, the release of tetracycline incorporated in the polymer was measured. The study was subdivided in three parts. The specific purpose of part I was to preliminarily determine the potential for retention and the possible bioavailability of tetracycline after treatment of single rooted teeth and molars with class two furcation (Hamp et al. 1975) defects. The purpose of part II was to explore if retention could be improved by modifying the clinical conditions at application time to minimize the presence of fluid on the root surface. The purpose of part III was to determine whether a small and controlled amount of polymer could be better retained within the pocket than an uncontrolled quantity overfilling the pocket.