
|
Photophysics and Photochemistry of Transition Metal Compounds |
| Home Research Members Collaborations Publications |

|
 |
|||||||
A comparison of the vibrational spectra of many inorganic borohydrides allows us to distinguish compounds with isolated BH4- ions and compounds containing complex ions such as Sc(BH4)4-. The characteristic spectral features of both types of compounds are identified, showing that the B–H bonding is quite different in both cases. A detailed analysis of the vibrations of the isolated BH4- ions provides new information about their local structure. Angular deformations of individual borohydride ion are analyzed quantitatively. It appears that the compounds containing isolated BH4- ions belong to those with the most electropositive cations and the highest decomposition temperature, while the complex borohydrides show significantly lower decomposition temperatures and possible diborane formation. | ||||||||
 |
|
|||||||
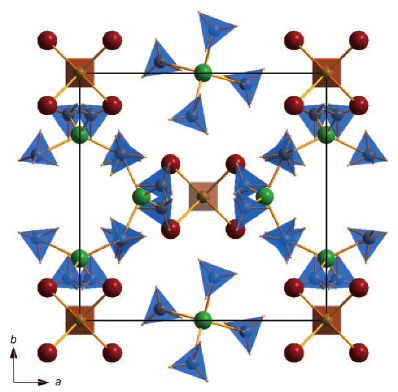
The new double-cation Al-Li-borohydride is an attractive candidate material for hydrogen storage due to a very low hydrogen desorption temperature (~70 °C) combined with a high hydrogen density (17.2 wt %). It was synthesised by high-energy ball milling of AlCl3 and LiBH4. The structure of the compound was determined from image-plate synchrotron powder diffraction supported by DFT calculations. The material shows a unique 3D framework structure within the borohydrides (space group=P-43n, a=11.3640(3) Å). The unexpected composition Al3Li4(BH4)13 can be rationalized on the basis of a complex cation [(BH4)Li4]3+ and a complex anion [Al(BH4)4]-. The refinements from synchrotron powder diffraction of different samples revealed the presence of limited amounts of chloride ions replacing the borohydride on one site. In situ Raman spectroscopy, differential scanning calorimetry (DSC), thermogravimetry (TG) and thermal desorption measurements were used to study the decomposition pathway of the compound. Al-Li-borohydride decomposes at ~70 °C, forming LiBH4. The high mass loss of about 20 % during the decomposition indicates the release of not only hydrogen but also diborane. | ||||||||
Download this list in format RIS
 EndNote
EndNote  BibTex
BibTex  PDF XML
PDF XML Last update Friday December 08 2017