
|
Photophysics and Photochemistry of Transition Metal Compounds |
| Home Research Members Collaborations Publications |

|
||||||||
The role of ligand-field states for the photophysical properties of d6 systems has been discussed in a large number of publications over the past decades. Since the seminal paper by Houten and Watts, for instance, the quenching of the 3MLCT luminescence in ruthenium(II) polypyridyl complexes is attributed to the presence of the first excited ligand-field state, namely a component of the 3T1(t2g5eg1) state, at similar energies. If this state lies above the 3MLCT state, the luminescence is quenched via thermal population at elevated temperatures only. If it lies well below, then the luminescence is quenched down to cryogenic temperatures. In this contribution we present transient absorption spectra on non-luminescent ruthenium polypyridyl complexes such as [Ru(m-bpy)3]2+, m-bpy = 6-methyl-2,2’-bipyridine, in acetonitrile at room temperature, which reveal an ultra-rapid depopulation of the 3MLCT state but a much slower ground state recovery. We propose that in this and related complexes the methyl groups force longer metal-ligand bond lengths, thus resulting in a lowering of the ligand-field strength such that the 3dd state drops to below the 3MLCT state, and that furthermore the population of this state from the 3MLCT state occurs faster than its decay to the ground state. In addition we demonstrate that in this complex the luminescence can be switched on by external pressure, which we attribute to a destabilisation of the ligand-field state by the pressure due to its larger molecular volume compared to the ground state as well as the 3MLCT state. | ||||||||
|
||||||||
Two new ethynylbipyridine-linked mono- and bis-tetrathiafulvalene (TTF) derivatives, together with a Ru(II) complex, were synthesized using Sonogashira coupling reactions and characterized by UV/vis spectroscopy and cyclic voltammetry. They display a clear electrochemically amphoteric behavior consisting of two reversible single-electron oxidation waves (typical for TTF derivatives) and one reversible single-electron reduction wave (bpy) and act as donor–acceptor (D–A) systems. Furthermore, for the Ru(II) complex, a quite intense fluorescence originating from the 3MLCT state is observed. | ||||||||
|
 |
|||||||
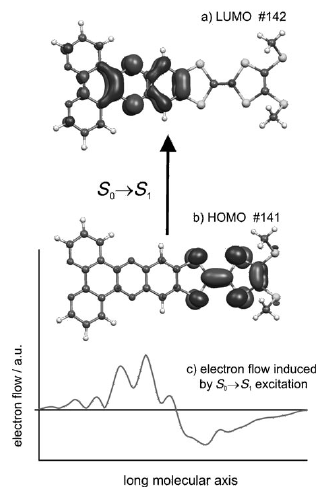
To study the electronic interactions in donor-acceptor (D-A) ensembles, D and A fragments are coupled in a single molecule. Specifically, a tetrathiafulvalene (TTF)-fused dipyrido[3,2-a:2',3'-c]phenazine (dppz) compound having inherent redox centers has been synthesized and structurally characterized. Its electronic absorption, fluorescence emission, photoinduced intramolecular charge transfer, and electrochemical behavior have been investigated. The observed electronic properties are explained on the basis of density functional theory. | ||||||||
Download this list in format RIS
 EndNote
EndNote  BibTex
BibTex  PDF XML
PDF XML Last update Friday December 08 2017