Over the past decades, liquid interfaces (air/liquid and liquid/liquid) have attracted considerable attention in different fields of science, where they play a crucial role. The bulk liquid phase, where molecules are randomly oriented, can be characterized by isotropic properties such as viscosity or dielectric constant. The situation is totally different at liquid interfaces, where the asymmetry of forces leads to an anisotropy of molecular orientation and hence to totally different properties. However, the measurement of these new features remains a major problem, and therefore, despite the numerous studies on liquid interfaces, our current knowledge is still relatively small. A major difficulty for experimentalists is to discriminate the interfacial region from the bulk phase, because, in conventional optical spectroscopies, the response from the interface, which consists of a few molecular layers, is totally buried in that originating from the bulk. The common approach to circumvent this problem is based on the measurements of second order nonlinear response, which is, within the dipolar approximation, zero in the bulk and thus arises from the interfacial region only. Such response can be performed, for instance, by surface second-harmonic generation (SSHG) technique, which have been widely used to probe stationary properties of interfaces. SHG signal can be resolved in time giving an opportunity to observe an interfacial excited-state dynamics. For example, we have investigated the excited-state dynamics of Malachite Green at air/liquid and liquid interfaces. This excited state lifetime of MG is known to depend strongly on the local viscosity and our time-resolved SSHG measurements have allowed the friction to be determined at interfaces (See picture).
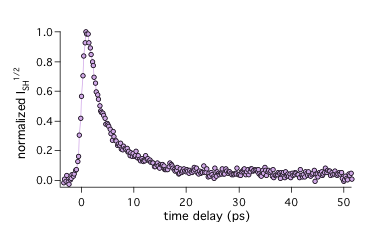
Similarly we have explored the effect of sodium salts added in the aqueous phase and found that, depending on the anion, these salts were favoring to a different extent aggregation of MG at the interface. We have shown that such measurements allow the interfacial concentration of anions (Cl-, SCN-) to be determined.
Contact:
Eric Vauthey
Physical Chemistry Department - Sciences II - University of Geneva
30, Quai Ernest Ansermet - CH-1211 Geneva 4 (Switzerland)
© All rights reserved by Eric Vauthey and the University of Geneva
Design and code by Guillaume Duvanel

