-
Search for Electron Delocalization from [Fe(CN)6]3- to the Dication of Viologen in (DNP)3[Fe(CN)6]2 · 10H2O
Ahmed S. Abouelwafa, Andreas Hauser, Valeriu Mereacre, Yanhua Lan, Gary J. Long, Fernande Grandjean, Gernot Buth, Christopher E. Anson and Annie K. Powell
Inorganic Chemistry, 56 (11) , 2017, p6477-6488


DOI:10.1021/acs.inorgchem.7b00540 | unige:94916 | Abstract | Article HTML | Article PDF | Supporting Info
|
| 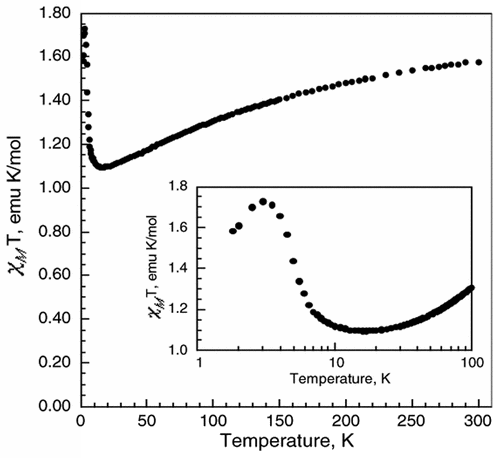  |
K3Fe(CN)6 reacts with the viologen 1,1′-bis(2,4-dinitrophenyl)-4,4′-bipyridinium dication, (DNP)2+, to form a supramolecular complex, (DNP)3[Fe(CN)6]2·10H2O (1). The crystal structure of 1 reveals that there are two [Fe(CN)6]3– anions within an organic framework of three (DNP)2+ cations with the shortest Fe(III)···Fe(III) distances of ca. 9.8 Å, distances that minimize extensive long-range magnetic exchange coupling interactions between the [Fe(CN)6]3– anions, and, thus, 1 is paramagnetic above ca. 17 K and exhibits weak ferromagnetic coupling between 17 and 3 K and antiferromagnetic coupling between 3 and 1.8 K. The long Fe(III)···Fe(III) distances permit slow spin–spin and slow spin–lattice paramagnetic relaxation, relative to the iron-57 Larmor precession frequency, as is evidenced by the Mössbauer spectra measured between 3 and 60 K; between 85 and 295 K, rapid paramagnetic relaxation is observed. Both the slow spin–spin and slow spin–lattice relaxation are mediated by the organic, π-conjugated viologen cations. The Fe–C distances, the Mössbauer isomer shifts, the temperature dependence of the magnetic susceptibility, and the 3 K magnetization results all indicate the presence of low-spin Fe(III) ions in the [Fe(CN)6]3– anions in 1. There is no unequivocal indication of the presence of any formal electron delocalization or transfer from the [Fe(CN)6]3– anion to the (DNP)2+ cations in the results obtained from X-ray crystallography, magnetic measurements, and Mössbauer spectra. Because of enhancement of the spin–orbit coupling by the heavy-atom or -ion effect, the Fe(III) ions in the [Fe(CN)6]3– anions interact with the (DNP)2+ cations, causing them to fluoresce with increasing intensity upon cooling from 90 to 25 K when excited at 300 nm. The resulting luminescence of the viologen (DNP)2+ cation induced by the [Fe(CN)6]3– anions indicates the presence of significant mixing of the molecular orbitals derived from the [Fe(CN)6]3– anions and the molecular orbitals associated with the (DNP)2+ cations to yield bonding supramolecular orbitals in 1, a mixing that is also observed between 50 and 3 K in the temperature dependence of the isomer shift of 1. |



