Publication 263
-
E. Raluy, O. Pàmies, M. Diéguez, S. Rosset, A. Alexakis,
“Sugar-based phosphite and phosphoroamidite ligands for the Cu-catalyzedasymmetric 1,4-addition to enones”
Tetrahedron: Asymmetry 2009, 20, 2167-2172.
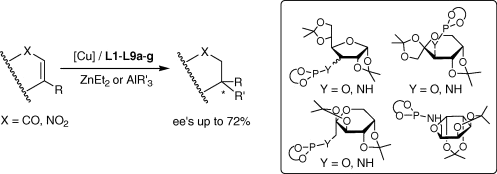
A modular sugar-based phosphoroamidite L1–L5a–g and phosphite L6–L9a–g ligand library was tested in the asymmetric Cu-catalyzed 1,4-conjugate addition reactions of β-substituted (cyclic and linear) and β,β′-disubstituted (cyclic) enones. The selectivity depended strongly on the configuration of carbon atom C-3, the size of the sugar backbone ring, the flexibility of the ligand backbone, the substituents and configurations in the biaryl phosphoroamidite moieties a–g, the type of functional group attached to the ligand backbone and the substrate structure. Therefore, by carefully selecting the ligand parameters, enantioselectivities of up to 60% for cyclic substrates and 72% for linear ones were achieved.
DOI : 10.1016/j.tetasy.2009.09.002
archive ouverte unige:8119
