Liste
Précédente Suivante
Publication 283
-
C. Hawner, A. Alexakis,
“Metal-catalyzed asymmetric conjugate addition reaction: formation of quaternary stereocenters”
Chem. Commun. 2010, 46, 7295-7306.

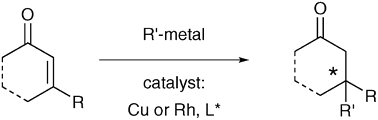
All-carbon quaternary stereocenters are ubiquitous motifs in biological products and pharmaceutical agents. However, due to sterical reasons, these centers are not always easily accessible. The metalcatalyzed conjugate addition reaction to trisubstituted conjugated substrates presents a viable methodology to create quaternary centers. In this article, different ways of activating the conjugate system towards nucleophilic addition will be described. An overview will be given on the different types of quaternary centers that are accessible through the conjugate addition reaction.
DOI : 10.1039/c0cc02309d
archive ouverte unige:12131
