Publication 339
-
H. Li, D. Grassi, L. Guénée, T. Bürgi, A. Alexakis,
“Copper-Catalyzed Propargylic Substitution of Dichloro Substrates: Enantioselective Synthesis of Trisubstituted Allenes and Formation of Propargylic Quaternary Stereogenic Centers”
Chem. Eur. J. 2014, 20, 16694-16706.
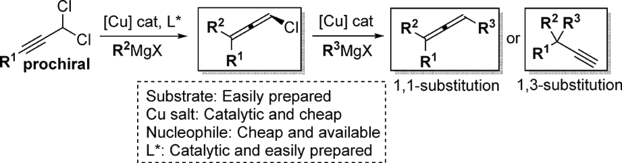
An easy and versatile Cu-catalyzed propargylic substitution process is presented. Using easily prepared prochiral dichloro substrates, readily available Grignard reagents together with catalytic amount of copper salt and chiral ligand, we accessed a range of synthetically interesting trisubstituted chloroallenes. Substrate scope and nucleophile scope are broad, providing generally high enantioselectivity for the desired 1,3-substitution products. The enantioenriched chloroallenes could be further transformed into the corresponding trisubstituted allenes or terminal alkynes bearing all-carbon quaternary stereogenic centers, through the copper-catalyzed enantiospecific 1,1/1,3-substitutions. The two successive copper-catalyzed reactions could be eventually combined into a one-pot procedure and different desired allenes or alkynes were obtained respectively with high enantiomeric excesses.
DOI : 10.1002/chem.201404668
archive ouverte unige:43540
