Liste
Précédente Suivante
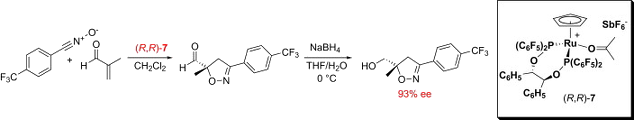
Publication 128
- “Chiral ruthenium Lewis acid-catalyzed nitrile oxide cycloadditions”
Y. Brinkmann, R.J. Madhushaw, R. Jazzar, G. Bernardinelli, E.P. Kündig,
Tetrahedron 2007, 63, 8413-8419.
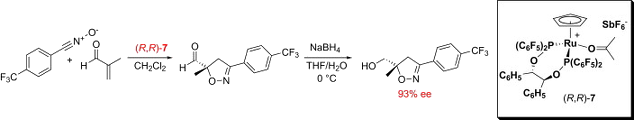
The synthesis of the chiral ligand (R,R)-BIPHOP-F is detailed. Its coordination to a cationic cyclopentadienyl ruthenium fragment generates [Ru (acetone)(R,R)-BIPHOP-F)Cp][SbF6], a transition metal Lewis acid that catalyzes the [3+2] dipolar cycloaddition reaction between aryl nitrile oxides and α,β-unsaturated aldehydes to give chiral 2-isoxazolines with yields of 43–98% and asymmetric purity of 60–93% ee. The stereochemistry of the major enantiomer is S, consistent with an approach of the nitrile oxide to the Cα-Si face of the enal in the anti-s-trans conformation in the catalyst site.
DOI : 10.1016/j.tet.2007.06.033
archive ouverte unige:7057
