Publication 131
- “Ruthenium–Lewis Acid Catalyzed Asymmetric Diels–Alder Reactions between Dienes and α,β-Unsaturated Ketones”
J. Rickerby, M. Vallet, G. Bernardinelli, F. Viton, E.P. Kündig,
Chem. Eur. J. 2007, 13, 3354-3368.
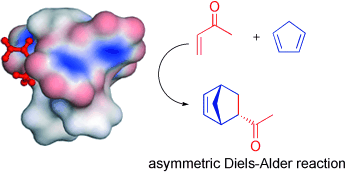
The complex [Ru(Cp)(R,R-BIPHOP-F)(acetone)][SbF6], (R,R)-1 a, was used as catalyst for asymmetric Diels–Alder reactions between dienes (cyclopentadiene, methylcyclopentadiene, isoprene, 2,3-dimethylbutadiene) and α,β-unsaturated ketones (methyl vinyl ketone (MVK), ethyl vinyl ketone, divinyl ketone, α-bromovinyl methyl ketone and α-chlorovinyl methyl ketone). The cycloaddition products were obtained in yields of 50–90 % and with enantioselectivities up to 96 % ee. Ethyl vinyl ketone, divinyl ketone and the halogenated vinyl ketones worked best and their reactions with acyclic dienes consistently provided products with >90 % ee. α-Chlorovinyl methyl ketone performed better than α-bromovinyl methyl ketone. The reaction also provided a [4.3.1]bicyclic ring system in 95 % ee through an intramolecular cycloaddition reaction. Crystal structure determinations of [Ru(Cp)((S,S)-BIPHOP-F)(mvk)][SbF6], (S,S)-1 b, and [Ru(Cp)((R,R)-Me4BIPHOP-F)(acrolein)][SbF6], (R,R)-2 b, provided the basis for a rationalization of the asymmetric induction.
DOI : 10.1002/chem.200600851
archive ouverte unige:7731
