Liste
Précédente Suivante
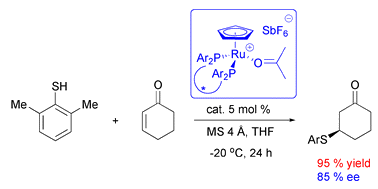
Publication 145
- “Asymmetric ruthenium-catalyzed 1,4-additions of aryl thiols to enones”
A. Bădoiu, G. Bernardinelli, C. Besnard, E.P. Kündig,
Org. Biomol. Chem. 2010, 8, 193-200.

Well defined, stable, one-point binding ruthenium complexes 1 and 2 selectively bind and activate α,β-unsaturated carbonyl compounds for cycloaddition reactions. These mild Lewis acids catalyze asymmetric 1,4-addition reactions of aryl thiols to enones with product selectivities up to 87% ee. 31P NMR experiments provide an insight into the intricate equilibria governing the reaction mechanism. The absolute configuration of the major products indicates enones to react in the syn-s-trans orientation. Models based on X-ray structures of the Ru complexes can be used to rationalize selectivity.
DOI : 10.1039/b918877k
archive ouverte unige:4864
