Publication 186
- “Palladium-N-Heterocyclic Carbene (NHC)-Catalyzed Asymmetric Synthesis of Indolines through Regiodivergent C(sp3)—H Activation: Scope and DFT Study”
D. Katayev, E. Larionov, M. Nakanishi, C. Besnard, E.P. Kündig,
Chem. Eur. J. 2014, 20, 15021-15030.
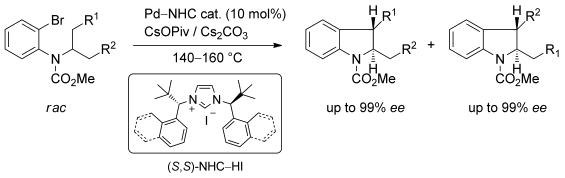
Two bulky, chiral, monodentate N-heterocyclic carbene ligands were applied to palladium-catalyzed asymmetric C—H arylation to incorporate C(sp3)—H bond activation. Racemic mixtures of the carbamate starting materials underwent regiodivergent reactions to afford different trans-2,3-substituted indolines. Although this CAr—Calkyl coupling requires high temperatures (140–160 °C), chiral induction is high. This regiodivergent reaction, when carried out with enantiopure starting materials, can lead to single structurally different enantiopure products, depending on the catalyst chirality. The C—H activation at a tertiary center was realized only in the case of a cyclopropyl group. No C—H activation takes place alpha to a tertiary center. A detailed DFT study is included and analyses of methyl versus methylene versus methine
DOI : 10.1002/chem.201403985
archive ouverte unige:41531
