Modelling lungs to limit testing on mice
The UNIGE is awarding its 3R prize to the creator of a cell model which, by reproducing a lung infection, has enabled the identification of a promising treatment.
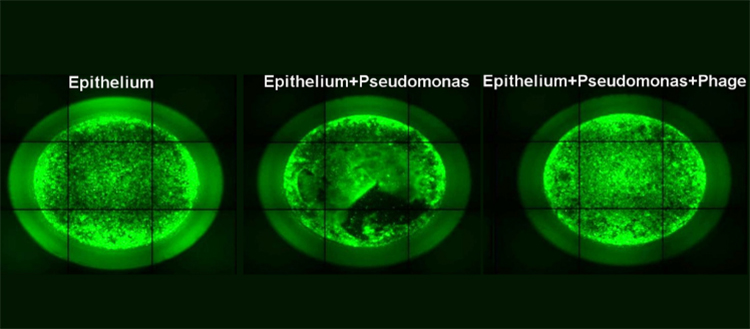
Fluorescent microscopy image of a human lung epithelium cell line model: alone, during infection by P. aeruginosa, after phage treatment. © UNIGE – Thilo Köhler
Pseudomonas aeruginosa is a bacterium responsible for many lung infections, particularly in people with cystic fibrosis or a weakened immune system. The emergence of resistance to most common antibiotics, which thus have become almost ineffective, makes it necessary to develop new therapies to combat these potentially fatal infections. Scientists at the University of Geneva (UNIGE) have developed an ex vivo model based on human lung cells to test the efficacy of several treatments: antibiotics, bacteriophages (viruses capable of infecting and destroying bacteria) or a combination of both. Accurate and close to clinical reality, this model has been awarded the UNIGE 3R prize, which since 2016 has distinguished a UNIGE researcher for their contribution to the 3R principles. The 3Rs principle – for “reduce”, “refine”, “replace” – aims to reduce the number of animals used in experimentation, to refine the methodology to minimise the constraints undergone by the animal while improving the quality of the results obtained, and to replace the animal model by alternative methods as soon as possible. This research can be discovered in an article published in August 2020 in the journal Frontiers in Microbiology.
“In order to make new discoveries and develop new therapies, scientific research relies on several methods of investigation. At UNIGE, any scientific project involving animal experimentation is evaluated in order to improve the welfare of the animals during experiments, to reduce the number of animals needed to achieve the goals of the project, and to replace the use of animals with alternative methods when possible. Our commitment to the 3Rs principle is strong and valued through the 3Rs Award”, underlines Daniele Roppolo, Director of Animal Experimentation at UNIGE. The UNIGE 3R Prize 2021 will be awarded on Tuesday 8 June 2021 at 5 pm, during the Prize Ceremony of the Faculty of Medicine’s Prize Commission, broadcasted online.
Countering antibiotic-resistant bacteria
Pseudomonas aeruginosa is widespread and responsible for many hospital-acquired infections, yet it remains one of the most difficult bacteria to treat. “The emergence of bacteria that are increasingly resistant to antibiotics is becoming a global scourge”, stresses Thilo Köhler, a researcher in the Departments of Microbiology and Molecular Medicine and of Medicine at UNIGE Faculty of Medicine, who led this work and is the winner of the UNIGE’s 3R prize. “It is therefore urgent to strengthen our therapeutic arsenal with other tools, including bacteriophages.”
Bacteriophages are viruses that specifically infect bacteria and can therefore be of interest to fight bacterial infections. Known for nearly a century, their use has however fallen into disuse. Their therapeutic interest has only recently resurfaced, with the appearance of multi-resistant bacteria and the lack of new antibiotics to combat them. Phages are indeed active on multi-resistant bacteria and have the advantage of being specific to a single type of bacteria, thus sparing our microbiota. In addition, phages are self-regulating depending on the presence or absence of the bacteria. “However, they can also cause resistance in bacteria”, says Thilo Köhler. “We therefore decided to test them alone and then in combination with antibiotics, with the idea of discovering the most effective treatment that is least likely to trigger these defence mechanisms.” Until now indeed, phagotherapies had only been tested for periods of less than 24 hours; their longer-term effects, as well as their effect on the host immune system, had never been studied in the context of P. aeruginosa infection.
A cellular model as close as possible to a real lung
To test these potential treatments, the scientists used an ex vivo model of human lung epithelial cell cultures, whose function is to form a protective barrier and ensure the hydration of mucus. Two types of cells were used: healthy cells and cells with a mutation in the cftr gene, which causes cystic fibrosis.
“Building on an existing technology, we created a new model adapted to a pulmonary setting in the context of a Pseudomonas infection”, explains Thilo Köhler. “In addition, we used cells that reproduce cystic fibrosis in order to establish whether the treatment we propose could also effectively treat lung infections affecting these patients.” Moreover, the evaluation of the efficacy of a phage/antibiotic combination in an animal model carried out by another team validates the ex vivo model using human cells: it is just as effective, while coming as close as possible to the clinical reality. It could therefore be adapted to other types of lung infection to study the effectiveness of new treatments without having to use animals.
Combining drugs for better treatment
When infected with P. aeruginosa, lung epithelial cells are rapidly unable to maintain the protective epithelial barrier. When treated with different types of phages or with an antibiotic (ciprofloxacin) a few hours after infection, the epithelial barrier remains impermeable. The effect is therefore present, but not definitive: 72 hours later, the bacteria have indeed started to multiply again, indicating the existence of a resistance mechanism.
On the other hand, when the two treatments are combined, the epithelial cells are much better protected: most of the bacteria disappear and the few survivors have lost their dangerousness. “Our results are positive in the healthy cell model as well as in the model using cells with a cftr gene mutation, which is very good news!” Thilo Köhler is pleased to say. Moreover, this type of treatment does not induce an inflammatory reaction, showing that it is highly acceptable to the organism, which does not seek to defend itself against the drug. This study therefore demonstrates the effectiveness of such a treatment, which will have to be confirmed in future clinical studies.
