Towards immunotherapies with fewer side effects
By identifying the causes of neurotoxicity shown by an immunotherapy used against childhood leukaemia and lymphoma, an American-Swiss team is developing a new generation treatment without side effects.
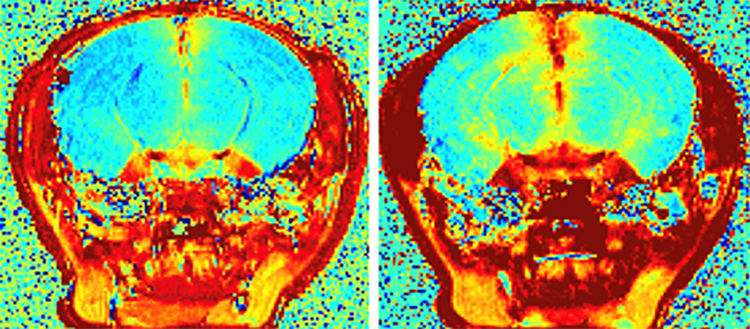
CD19 CAR-T cells induced BBB leakage in mice brains, recapitulating the neurotoxicity observed in humans. @UNIGE
In recent years, immunotherapies, which aim to stimulate the immune system of people suffering from difficultly treatable cancers to detect and destroy tumours, have been offering new hope to patients. CAR-T are genetically modified immune cells recently approved for the treatment of childhood leukaemia and lymphoma. This treatment is generally highly effective and not harmful, but sometimes comes with a risk: in rare cases, patients suffer from severe neurological disorders that can be life-threatening. A team from the University of Geneva (UNIGE), the University Hospitals of Geneva (HUG), in Switzerland; the University of Pennsylvania, Stanford University, and the Parker Institute for Cancer Immunotherapy in the United States has succeeded in identifying one possible cause of this neurotoxicity. The target molecule of CAR-T cells is indeed present not only on the surface of cancer cells, but also on the surface of pericytes, cells whose role is to protect the brain from external invasion. Their destruction by CAR-T cells could therefore explain why some patients suffer from neurotoxic effects. This major discovery, to be read in the journal Cell, will hopefully allow scientists to modify the treatment to avoid the neurotoxic effect while maintaining its anti-tumor power.
CAR-T, that stands for «chimeric antigen receptor», are human immune cells genetically reprogrammed to detect a target molecule, expressed on the surface of cancer cells. CAR-Ts are thus able to recognize and eliminate diseased cells. Approved in 2017 for the treatment of certain acute leukemias in children and certain lymphomas resistant to other treatments, this immunotherapeutic strategy is highly effective in achieving complete remissions of up to 90% of patients. However, potentially severe cases of neurotoxicity may arise: 30 to 50% of patients will experience transient neurological disorders (e.g. difficulty walking or speaking), severe epileptic seizures, or in rare cases, comatose states that can prove fatal.
To understand why, Denis Migliorini, assistant professor at the Centre for Translational Research in Onco-haematology (CRTOH) of UNIGE Faculty of Medicine, holder of the ISREC Chair in Brain Tumor Immunotherapy and Clinical Academic physician at the HUG, has joined forces with scientists at the University of Pennsylvania and with specialists in single cell genomics at Stanford University. «We suspected that the CD19 marker could be expressed by healthy brain cells, which would explain the brain damage that some patients suffer from. But which cells?» explains Denis Migliorini. «To our great surprise, the large-scale genetic analyses we conducted revealed the presence of CD19 on the surface of some human brain pericytes.»
Pericytes are a cell population responsible for maintaining the integrity of the blood-brain barrier that protects the brain from damaging molecules. When expressing CD19, pericytes turn into targets for destruction.
Better targeting of tumour cells
To confirm the validity of this discovery, scientists used mouse models with which the neurotoxicity observed in patients was reproduced. «The next step, which is already underway, is to modify the CARs. By adding genes that give them the ability to recognize pericyte markers in addition to tumor markers, our new CAR-T cells will hopefuly be able to kill tumour cells but spare the pericytes,» explains Denis Migliorini. The treatment would therefore no longer trigger a neurotoxic response, but would retain its efficacy.
In addition to leukaemia and lymphoma, the researchers are also trying to extend the effectiveness of CAR-T treatments to other «solid» cancers. However, whereas leukaemia and lymphomas have a clearly identified target with the CD-19 marker, solid tumours, and in particular brain tumours, are made up of different cell populations each expressing different markers. The aim is therefore to develop CAR-Ts that are capable of attacking different cell markers while remaining insensitive to those of healthy cells.
The CRTOH is part of the Swiss Cancer Center Léman (SCCL), a multidisciplinary alliance bringing together UNIGE, HUG, EPFL, CHUV, UNIL and the ISREC Foundation to conduct fundamental, translational and clinical research in the field of cancer.
24 Sept 2020
