Mechanisms of ubiquitination
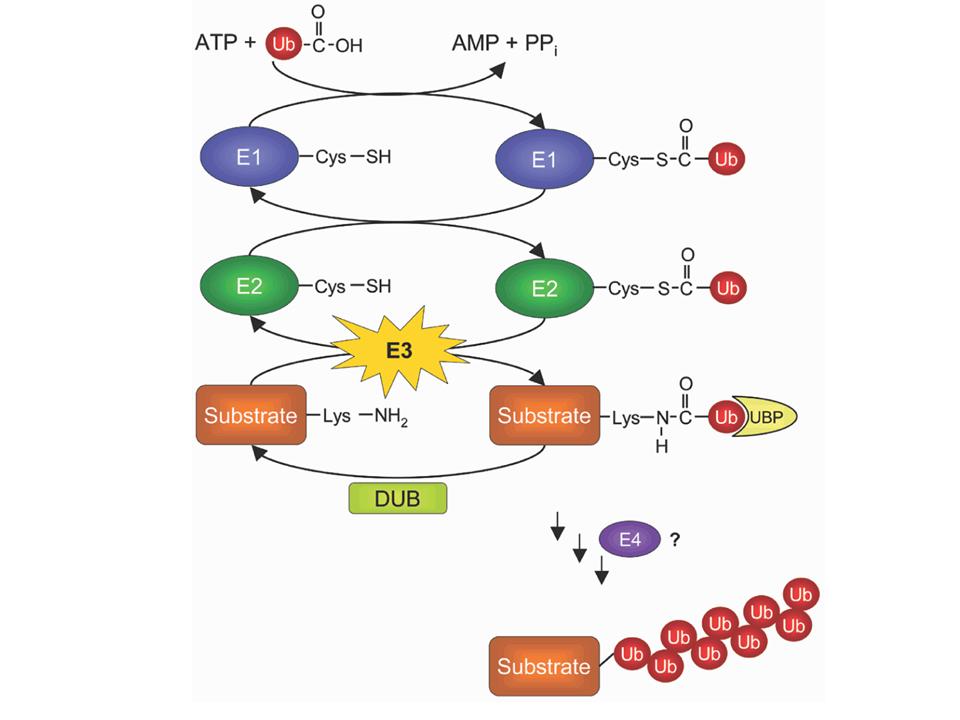
1) The unique E1 "ubiquitin-activating" enzyme activates ubiquitin by
binding it to one of its own cysteins.
2) It then passes ubiquitin onto one among several dozens of E2
"ubiquitin-conjugating" enzymes. Here again, the C-terminus of
ubiquitin is bound via a cystein.
3) One among hundreds of E3 "ubiquitin ligase" enzymes transfers
ubiquitin to its final protein target. The C-teminus of ubiquitin is
linked to the NH2 side chain of a lysine in the target, forming a
covalent peptide bond.
4) Because ubiquitin itself contains 7 lysines, it is possible to build
chains of ubiquitins onto the target protein. This may require the same
or a different E3, and possibly the help of an E4 enzyme.
5) Specialized enzymes known as de-ubiquitinases (DUB) can shorten the
chains, or even remove all ubiquitins from the target protein.
Fate of ubiquitinated proteins
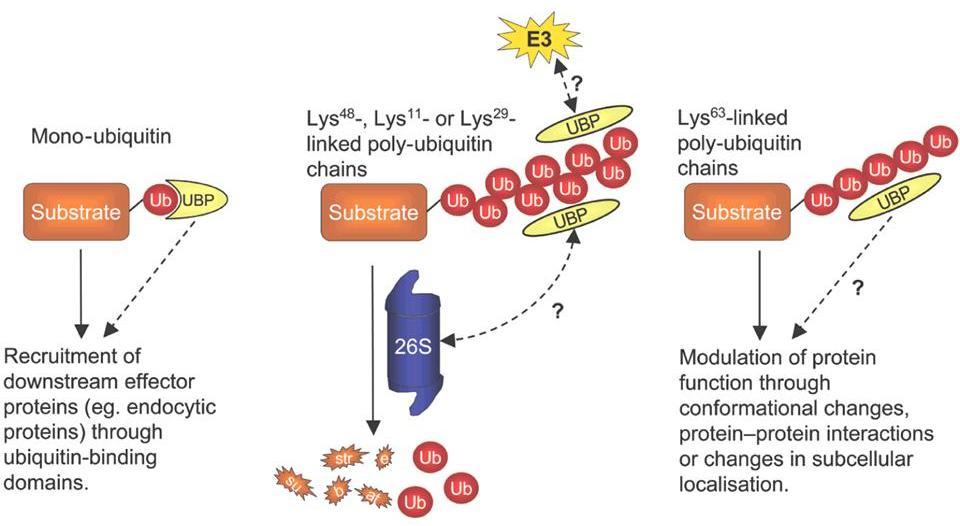
Depending on the number of ubiquitin moeties, and on the type of chains
(i.e. which one of the 7 lysines in ubiquitin is used to grow the
chain), the effect on the target protein may differ widely.
Some chains, mainly those using lysine 48 in ubiquitin, tag the target
protein for degradation in the 26S proteasome, as soon as the chain
grows beyond 4 ubiquitins.
But other chain types, as well as mono-ubiquitination, can serve to
modulate the activity of the target protein.
Source
Getting into position: the catalytic mechanisms of protein
ubiquitylation.
by L.A. Passmore & D. Barford
Biochemical Journal 379:513-525 (2004)

