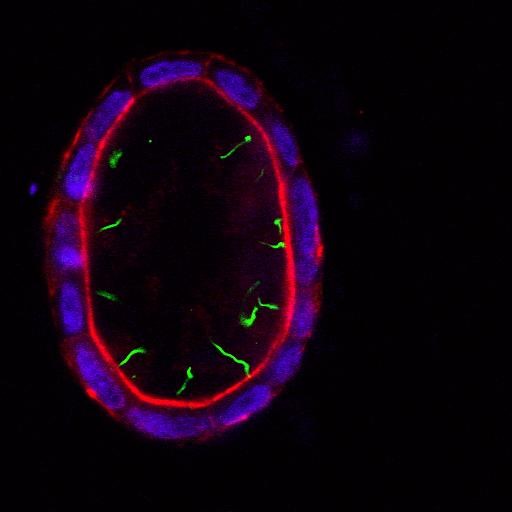
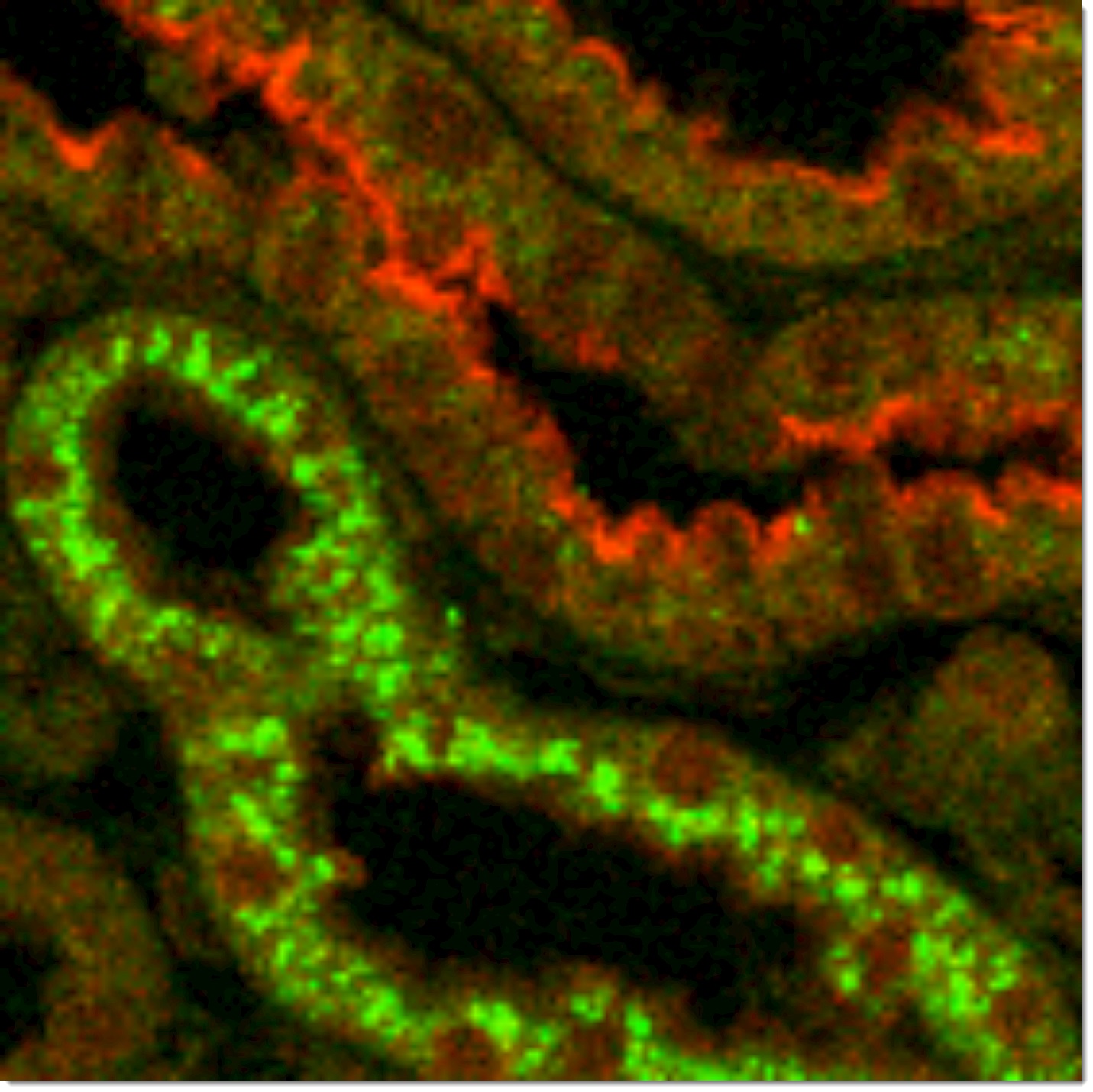
Laboratory of physiology and pathophysiology of kidney cells
My laboratory is working on renal physiology and pathophysiology. We are specialized in the study of ion transport and energy consumption by kidney tubule epithelial cells as well as the role of adrenal steroids in the progression of chronic kidney disease and kidney interstitial fibrosis.
We are currently studying the role of :
- increased metabolic work and energy consumption of remaining kidney tubule epithelial cells
- increased aldosterone secretion in response to high dietary potassium intake
- increased tissular conversion of inactive cortisone to active cortisol