Cooperation of two proteins for calcium flux
The STIM1 protein mediates the Store Operated Calcium Entry SOCE pathway. This pathway controls key physiological processes, such as the proliferation of immune cells. Following a calcium depletion of the endoplasmic reticulum, the cellular calcium storage compartment, STIM1 undergoes a series of conformational changes that open cell membrane channels, causing an influx of calcium into cells. The entering calcium activates essential cell signaling circuits and gene expression programs as illustrated by the severe combined immunodeficiency and muscle weakness of patients bearing mutations in STIM1.
The cooperation between UNC93B1 and STIM1
Scientists from the laboratory of Prof. Nicolas Demaurex revealed a few years ago in their publication in Nature Communications that activation of STIM1 relies on its interaction with another protein called UNC93B1. This study highlighted an important cooperation that prompted researchers to understand more precisely how these two proteins interact.
Unraveling the mystery of their cooperation
In her recent study in the Journal of Biological Chemistry, Dr Wen-An (Jennifer) Wang from the laboratory of Prof. Nicolas Demaurex has elucidated the key mechanisms of their interaction. She found that UNC93B1 does not bind anywhere on STIM1, but to the part of STIM1 that crosses the membrane of the endoplasmic reticulum (as illustrated on the left of the figure below). Her in vitro experiments also revealed that UNC93B1 binding promotes the elongation and dimerization of STIM1 (right side in the figure below), a critical preliminary step in the initiation of SOCE.
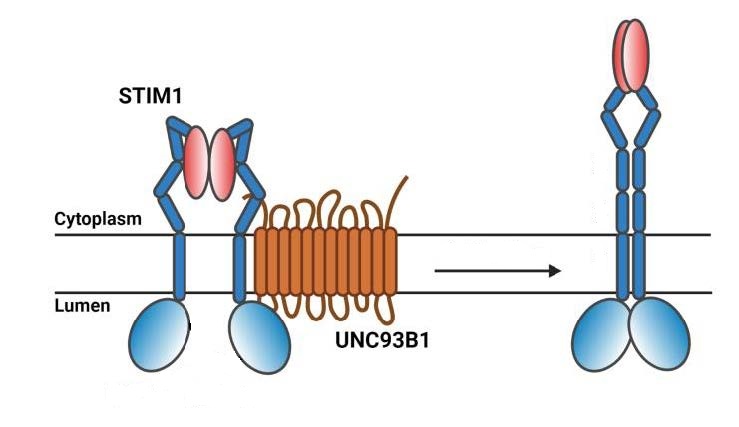
When calcium is lacking, UNC93B1 binding to STIM1 (left configuration) promotes its elongation and dimerization (right configuration), a critical preliminary step in the initiation of SOCE. © adapted from Figure 6 in Wang & Demaurex JCB 2022.
What’s next?
By controlling the initial steps of the STIM1 activation cascade, UNC93B1 can act as a gatekeeper to modulate STIM1 activity in specific contexts. The research team now aims to determine the physiological role of this interaction in immune cells, known to rely on UNC93B1 to recognize molecules derived from microbes.
10 Feb 2022
