Research
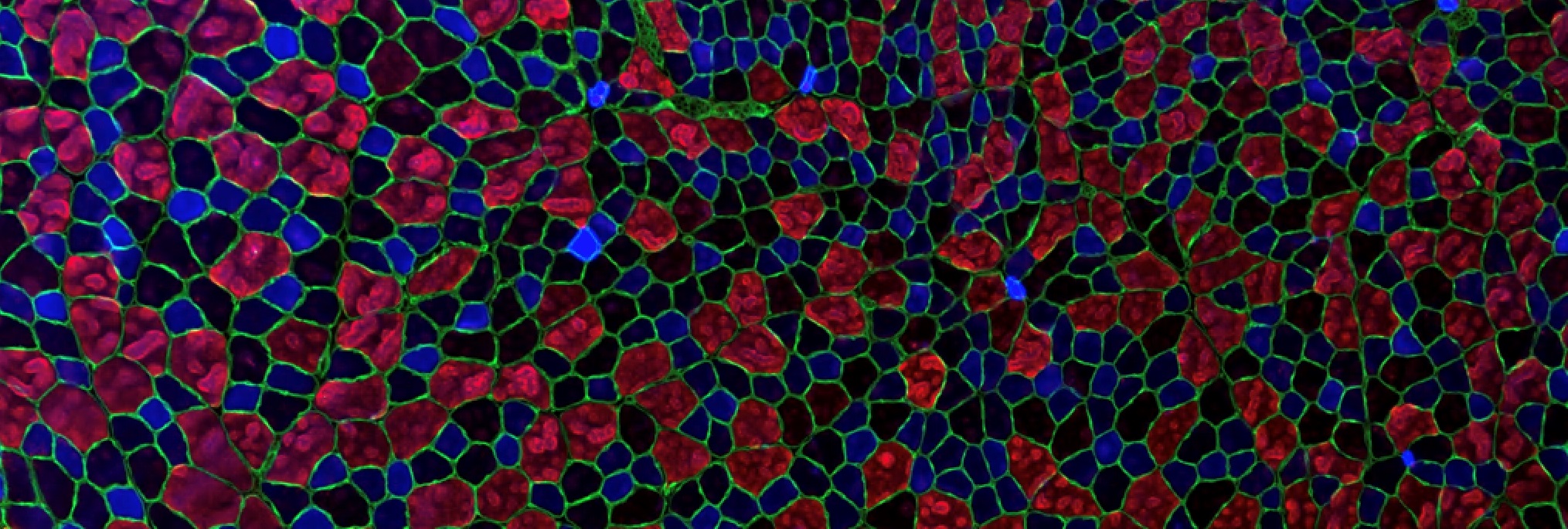
Skeletal muscle cells are very large cells (up to several tens of centimeters), multi-nucleated and unable to divide.
Following muscular lesions, such as those caused by intense physical exercise, the tissue is able to repair itself thanks to the presence of satellite cells, the muscle stem cells present in adults. These satellite cells are usually quiescent, but after an injury, they become activated and proliferate to form myoblasts. This phase is initiated by tissue inflammation following injury. When the inflammation decreases, the myoblasts stop proliferating and begin to differentiate into muscle cells and then fuse to form immature myotubes.
The mechanisms involved are numerous and include the generation of calcium signals and the activation of transcription factors. This results in the synthesis of muscle-specific proteins, contractile apparatus and ion channels involved in excitation-contraction coupling. At the same time, the very complex internal organization of mature myotubes takes place.
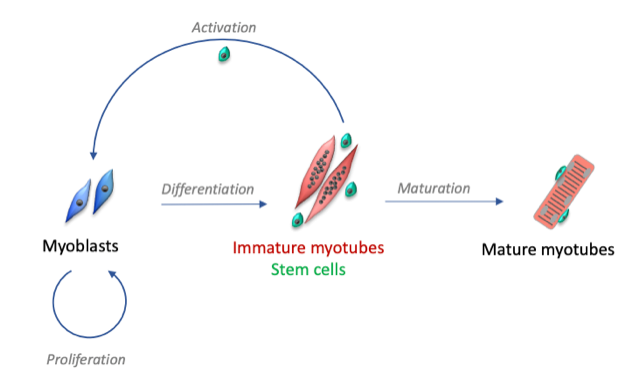
Our research group studies the mechanisms involved in the regeneration of human skeletal muscle, with two main research axes:
- Highlight the specific role of proteins involved in calcium signaling during the differentiation stages: transformation of myoblasts into immature myotubes, then maturation of myotubes.
- Determine the mechanisms that allow the activation, but also the maintenance or the return to a quiescent state of muscle stem cells. These mechanisms are essential to maintain the necessary muscle homeostasis throughout life, the alteration of these mechanisms leading to the loss of muscle mass and being at the basis of various myopathies.
