Research
G-protein coupled receptors (GPCRs) are versatile membrane receptors that translate binding of a diverse array of extracellular ligands into intracellular signaling cascades. As such, they play critical roles in human physiology and disease and are prominent therapeutic targets. We aim to understand how GPCRs function in the cellular context and more specifically, how receptors in different cellular organelles translate ligand-binding into precise signaling outcomes. We envision that a deeper understanding of the spatiotemporal logic of GPCR signaling on the level of individual cells is key to determining the specificity of drug action and may contribute to the development of safer therapeutics capable of selectively driving desired signaling responses.
Ongoing research projects
Receptor signaling from internal membrane
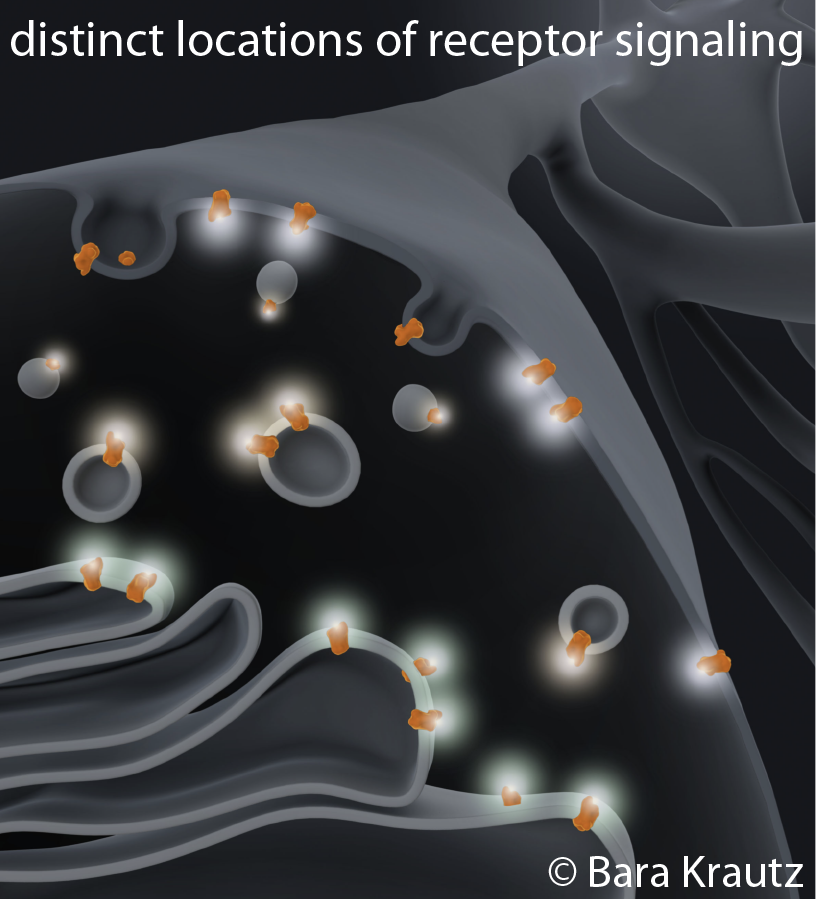
The classical model for GPCR function is restricted to signaling events that occur exclusively in the plasma membrane. This concept was recently challenged by an emerging body of data demonstrating that some GPCRs signal from intracellular organelles, which presents a new paradigm for spatially-encoded signaling bias. Several downstream effectors, including G proteins and adenylyl cyclases, are known to be abundant in intracellular organelles, however, the precise internal signaling events remain elusive and how they effect physiological processes is largely unknown.
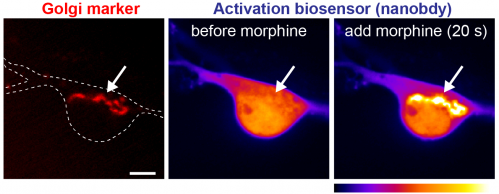
Focusing on physiologically and pharmacologically important opioid receptor ligands, we have previously shown that opioid drugs (e.g. morphine) differ from endogenous peptides (e.g. endorphin) in the cellular location at which they drive receptor activation. One exciting possibility is that some drug-specific effects and abuse responses may be explained by what cellular pool of receptors a certain drug is interacting with. We seek to unravel how receptor signaling in plasma membrane, endosomes, and Golgi apparatus contributes to opioid drug action. For this we combine live cell fluorescence microscopy, nanobody-based biosensors, signaling reporters, unbiased genomic approaches, and diverse pharmacological tools.
Regulation of internal receptor pools
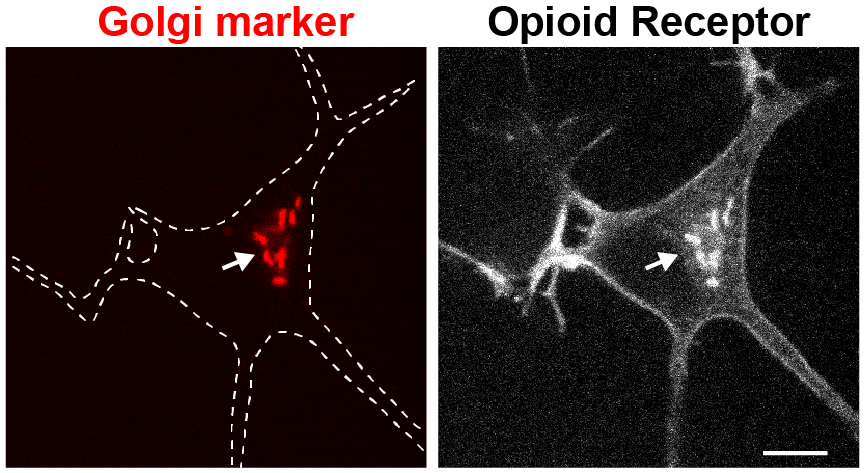
What regulates the abundance of GPCRs at internal organelles? Some GPCRs are inefficiently targeted to the cell surface and can be arrested in the secretory pathway. Varying levels of receptors in internal organelles may critically influence signaling outcomes in response to membrane-permeant ligands (e.g. drugs, hormones). We seek to identify proteins that regulate anterograde trafficking of GPCRs as they may be key factors to understanding the biological function of internal receptor pools. We will use an unbiased screening approach and cell biological / biochemical methods to learn about the exit and retention machineries that regulate GPCR trafficking.
Novel tools to sense and modulate GPCR function
Several studies have now established the usefulness of single-domain antibodies, termed nanobodies, for detecting target proteins in the intact cellular environment with high precision. Intracellularly expressed fluorescently labeled nanobodies can act as biosensors to detect location and conformational changes of proteins. However, due to their different binding modes and affinities, they can also serve as reagents to modulate target protein function. We will develop novel nanobody-based reagents that have the ability to sense or block receptor activity and anticipate to generate powerful tools with immediate applications in our own studies of location-encoded signaling. Beyond this, we seek to establish a toolkit to probe endogenous GPCR function and trafficking with high sensitivity and subcellular resolution in more complex biological tissues. In our work, we will combine protein biochemistry, image-based assays, and high-resolution microscopy techniques.