Tracking neurogliaform cells and their role in the cortical microcircuit

Studying neurogliaform-type interneurons involves the five key elements of stress-related pathologies: brain development, the prefrontal cortex, serotonergic pathway, interneurons and plasticity. Here’s how.
Experience constantly alters the activity of the brain and the structure of the neural networks. Known as activity-dependent plasticity, it is an essential process for memory and learning. Although it is especially pronounced during development, it continues throughout life. The underlying mechanisms of plasticity are particularly interesting for Synapsy since they play a role in most psychiatric illnesses (see boxed text on page 3). The group led by Anthony Holtmaat at the University of Geneva is studying the functional and structural aspects of this form of neural plasticity. Alexandre Dayer’s group, on the other hand, is trying to ascertain how neuronal activity and genetics can alter the developmental trajectories of neural cells during development and thus modify the connectivity of the neural networks. These are complementary approaches that lie at the heart of a collaborative project between the two laboratories involving Foivos Markopoulos, research fellow (maître-assistant) at the University of Geneva. The project aims to understand how a type of cortical interneuron called the neurogliaform cell regulates plasticity processes.
Serotonergic pathway singled out
But why study neurogliaform cells in particular? The answer comes from research carried out by Dayer’s laboratory on serotonin — a neurotransmitter involved in physiological functions such as sleep and mood. This research indicates that a dysfunction in the serotonergic pathway during brain development affects the development of cortical interneurons, such as the neurogliaform cell. In addition, Geneva-based researchers have identified that a serotonin receptor –HTR3A – influences the development of neurogliaform cells in the cerebral cortex. Interestingly, variation in the HTR3A gene has been associated to post-traumatic stress disorder (PTSD), which is studied in the cohort of Synapsy researcher Daniel Schechter (read the related article “Maternal exposure to violence and child psychopathology”). In addition, animal models that do not express the HTR3A receptor gene are unable to erase their fear, which is a typical PTSD trait.
Focusing on the mechanistic
Neurogliaform cells intrigue Foivos since they have been identified as the source of long-lasting inhibition “but to investigate their role in activity-dependent plasticity, we needed to be able to manipulate these cells with specificity”. For this reason, Markopoulos collaborated with Mathieu Niquille and Greta Limoni from the Dayer lab, who used genetic tools to trace the origins of 5-HT3AR-expressing interneurons. The scientists found that a specific pool of these interneurons comes from a different region than other types of interneurons, located outside the cerebral cortex in the preoptic zone. Furthermore, they travel long distances to reach the cortex a few days after birth and take several weeks to reach maturity. By investigating their electrophysiological and morphological profile, Markopoulos was able to identify these interneurons as the cortical neurogliaform cells.
With this information and the tools that have been developed, Markopoulos intends to investigate the specific role of neurogliaform cells in circuit mechanisms underlying activity-dependent plasticity. The aim is to have a full understanding of the role that these cells play in learning and behavior, and what the consequences are of a dysfunction of these mechanisms in the context of stress-related disorders. “We’re focusing on the circuit mechanisms that involve these cells. Since the activity of neurogliaform cells is likely to undergo negative regulation in the event of early exposure to stress, we hypothesize that this unbalances the modulation of synaptic activity mediated by these interneurons, with an impact on activity-dependent plasticity”, concludes Markopoulos.
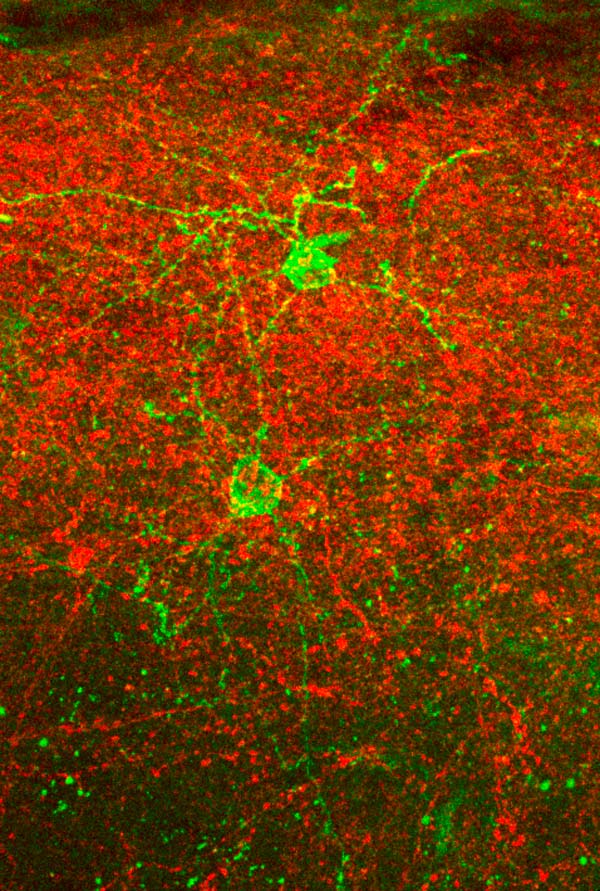
Neurogliaform cells (NGCs, green) and axons from the posteriomedial thalamus (red), expressing different light-activated ion channels, in barrel cortex layer 1. This combination allows for selective optogenetic activation of the one or the other element in order to study the inputs and outputs of NGCs. © Foivos Markopoulos, Holtmaat lab
10 Mar 2020