Lentivector production
Production of lentivectors is often achieved by transient transfection of the plasmid set into 293T cells by the calcium phosphate method (as shown in the diagram below).
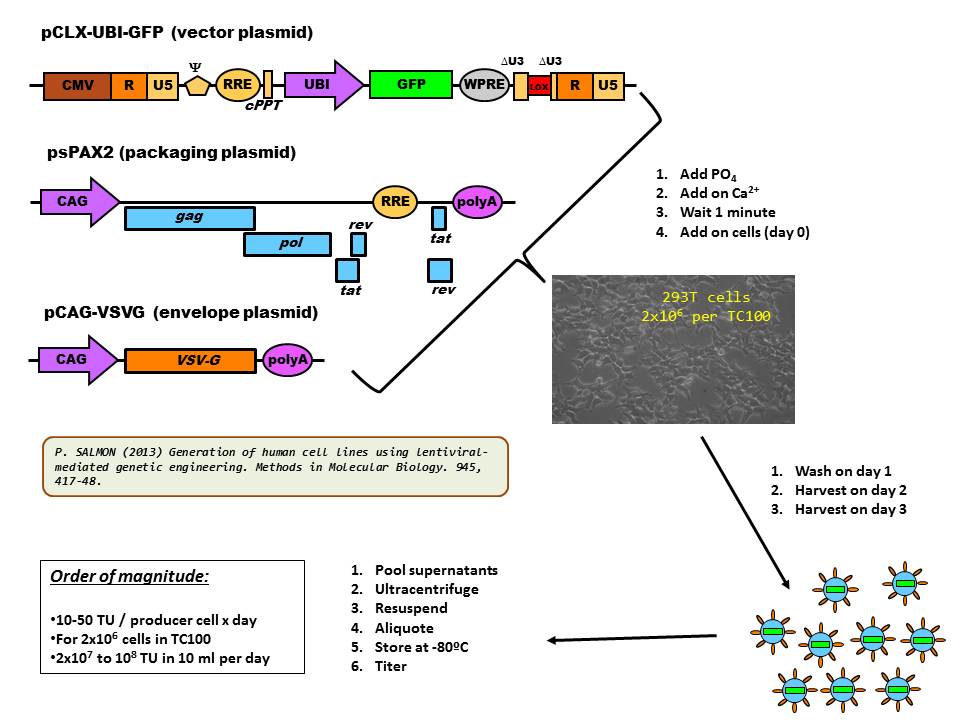
Since the Calcium Phosphate Technique is difficult to standardize and poorly reproducible from day to day or lab to lab, more and more labs are using the PEI (Poly-Ethylene-Imine) technique.
-
The Poly-Ethylene-Imine Technique (PEI)
This technique is very reproducible and is now used for small-scale (10 ml to 100 ml) to large-scale (1 liter to 50 liters) transfection, of either adherent or suspension 293 cells, in serum-containing or serum-free conditions.
Reagents
- All solutions and equipment coming into contact with living cells must be sterile, and aseptic techniques should be used accordingly.
- All maps and sequences of plasmids described here are available at https://www.unige.ch/medecine/vectorlab/
- Common plasmids for the generation of HIV1-based lentivectors can be obtained from Addgene http://www.Addgene.org.
- Use ultrapure or double-distilled water in all recipes.
- Producer cells: 293T/17 cells (from ATCC Cat# CRL-11268).
- D10 medium: Dulbecco’s modified Eagle medium (with 4.5 g/l glucose, glutamine, and pyruvate, Invitrogen Cat# 41966052 or equivalent) supplemented with antibiotics and 10% FBS.
- TE buffer: 10 mM Tris–HCl – 1 mM EDTA, pH 8.0. Used to redissolve all plasmids.
- Envelope plasmid: pCAG-VSVG or pMD2G dissolved at 1 mg/ml in TE buffer.
- Packaging plasmid: psPAX2 (encoding HIV-1 Gag, Pol, Tat, and Rev proteins) dissolved at 1 mg/ml in TE buffer.
- Vector plasmid: pFUGW dissolved at 1 mg/ml in TE buffer.
- 75% Ethanol in a spray bottle.
- PBS, pH 7.4.
- 0.25% Trypsin/EDTA.
- Sterile endotoxin-free 0.15 M NaCl solution
- 13–14% Bleach solution (w/v).
- 10-cm tissue culture dishes.
- 37°C humidified incubators, 5% CO2.
- 1.7 and 2.2-ml and microcentrifuge tubes, sterile, disposable.
- 15- and 50-ml conical centrifuge tubes, sterile.
- 50 ml syringes ml and 0.45-mm pore size PVDF filters.
- PEG-it Virus precipitation solution, SBI Cat# LV825A-1
- PEI Preparation (1 mg/mL)
Materials:
- 1g PEI 25K (Polysciences Catalog# 23966)
- 1L Milli-Q® water, water for injection (WFI), or comparable biological-grade water
- 12 M Hydrochloric Acid (HCl)
- 10 M Sodium Hydrochloride (NaOH)
- Disposable 0.1-0.2μm PES vacuum sterile-filter.
- Sterile HDPE or polypropylene storage vials for reagent aliquots.
Equipment:
- 1L glass beaker
- 1L glass graduated cylinder
- Calibrated pH meter
- 2 x disposable 1mL plastic pipettes
- Stir plate
- PTFE coated stir bar
- Vacuum pump
Method:
- Dispense 1g of PEI 25K into beaker and suspend in 900 mL of water.
- Add stir bar and set stirring to produce a small vortex.
- Add hydrochloric acid dropwise until pH is < 2.0.
- Cover top of beaker and stir for up to 3 hours until powder fully dissolves.
- Add sodium hydroxide dropwise until pH is 6.9-7.1.
- Transfer solution to graduated cylinder and add water until the total volume is 1L.
- Sterile-filter through vacuum membrane.
- Aliquot as desired and store at -20°C.
- Frozen aliquots can be stored at -20°C for up to one year. Aliquots can be thawed and kept at 4°C for up to two weeks, but should never be re-frozen.
Protocol
- Maintain 293T cells in D10 medium, in 10-cm tissue culture dish in a 37°C humidified incubator with a 5% CO2 atmosphere, and split them at ratio 1:10 using Trypsin/EDTA, three times per week (e.g., every Monday, Wednesday, and Friday). Frequent passages and keeping the 293T as individual cells will ensure high transfection efficiency.
- The day before the transfection, seed 3 dishes at 1.5 to 2.5 million cells per dish (10 cm). Cells must be approximately 1/3 confluent on the day of transfection.
- Incubate overnight in a 37°C humidified incubator with a 5% CO2 atmosphere.
- On the following day, co-transfect the cells according to the following recipe.
Envelope plasmid pCAG-VSVG 12 µg
Packaging plasmid psPAX2 24 µg
Vector plasmid pFUGW 24 µg
The vector plasmid (pFUGW given as example above) can be second or third generation since the psPAX2 plasmid provides Tat protein.
- Mix the plasmids in 2.2 ml Eppy and pipet up and down 20 times
- Add up to 750 µl of NaCl 0.15 M and pipet again up and down 20 times
- In a separate 1.7 ml Eppy add 180 µl of PEI solution to 570 µl of NaCl 0.15 M. Mix by vortexing.
- Add the PEI mix on the plasmid mix (in this order) and vortex mix top speed for 20 seconds.
- Leave the precipitate form at room temperature for 20–30 min.
- For each TC100, add 500 µl of precipitate dropwise to the cells. Mix by gentle swirling.
- Place the dish overnight in a 37°C humidified incubator with a 5% CO2 atmosphere.
- In the afternoon the next day, collect the supernatants.
- Transfer and pool the supernatants from the identical 3 plates into one 50-ml centrifuge tube.
- Filter the 30 ml of pooled supernatant with a 50 ml syringe connected to a 0.45 mm PVDF disk filter. Collect in a new 50-ml centrifuge tube.
- At that stage, you must have around 28 ml of unconcentrated supernatant.
- To this 28 ml of unconcentrated supernatant, add 8 ml of PEG-it, mix thoroughly but gently and without vortex and store at +4°C overnight and up to 4-5 days.
- Spin at 1500 g for 30 minutes. Aspirate as much supernatant without disturbing the pellet.
- Add 200 µl of cold PBS on the pellet and gently resuspend without making bubbles. I pipet up and down 30 times.
- Aliquote in 20 µl aliquots and store at -80°C
If you still want to get the technical details of the Calcium Phosphate technique, you can read from one of our bookchapters, by clicking on the picture below.

