Research Background & Membrane remodeling
Many proteins interact transiently with lipids membranes within cells, and modify their shape and size. They can also fuse, break and modify the composition of these membranes. But membranes are very peculiar objects, as they are visco-elastic 2D fluids that can auto-seal if broken.
We are interested in understanding how these special mechanical properties have constrained the way proteins remodel cellular membranes.
Eukaryotic cells and their compartments are separated from their environment by lipid membranes that are impermeable to most of the compounds degraded or produced through their metabolism. Most of the exchanges of material between these compartments are made through membrane intermediates that bud out of the lipid bilayers.
These intermediates are formed in three steps:
- The membrane is deformed by assemblies of proteins and lipids
- Cargo proteins and lipids are sorted within the bud
- The neck of the bud is broken to separate the donor membrane from the membrane intermediate, allowing its transport through the cell (see figure 1)
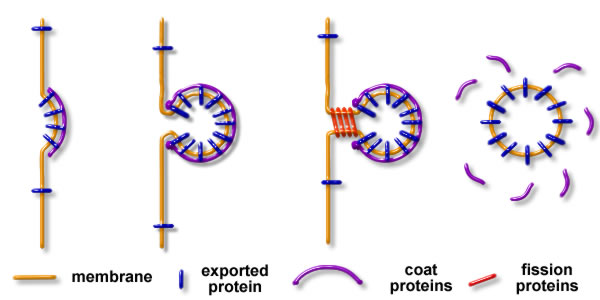 Figure 1: The 3 steps of the formation of a membrane intermediate: 1 membrane budding – 2 sorting of cargo components – 3 fission of the bud, and release of the free intermediate.
Figure 1: The 3 steps of the formation of a membrane intermediate: 1 membrane budding – 2 sorting of cargo components – 3 fission of the bud, and release of the free intermediate.
This last step is called membrane fission. It is a required step in many cellular processes that involve membrane remodeling. For example, membrane fission is required for some several stages of cell division and compartment maintenance (Golgi, mitochondria and reticulum multiplication through the cell cycle).
After three decades of research in the field of membrane traffic, many proteins having a role in one of these three steps have been identified: my research focuses on the mechanisms used by proteins and lipids to deform, sort and break membranes during the formation of transport carriers. Researchers have exhaustively identified each factor and most of their exact function.
But essential questions on how these proteins and lipids mechanically coordinate to create and sustain the shape and size of membrane carriers are still unsolved. Our goal is to use tools and concepts coming from soft-matter physics in order to quantitatively measure forces, energies and sizes generated by proteins in interaction with membranes. This will enable us to discriminate between different mechanisms that have been proposed in the past to explain how proteins remodel membranes.
