Liste
Précédente Suivante
Publication 167
- “Asymmetric Synthesis of (+)-Vertine and (+)-Lythrine”
L. Chausset-Boissarie, R. Arvai, G. Cumming, L. Guénée, E.P. Kündig,
Org. Biomol. Chem. 2012, 10, 6473-6479.

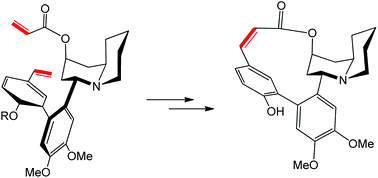
The total syntheses of the Lythracea alkaloids (+)-vertine and (+)-lythrine are described. Enantioenriched pelletierine is used as a chiral building block and engaged into a two step pelletierine condensation leading to two quinolizidin-2-one diastereomers in a 8:1 ratio. The major product is used in the synthesis of (+)-vertine via aryl–aryl coupling and ring closing metathesis to provide a Z-alkene α to the lactone carbonyl function. The same procedure was used for (+)-lythrine after base induced epimerization of the main quinolizidin-2-one diastereomer. Alternative classical ring closure strategies like macrolactonisation or aryl–aryl coupling failed.
DOI : 10.1039/C2OB25880C
archive ouverte unige:21968
