- Regiodivergent synthesis of pyrazino-indolines vs. triazocines via α-imino carbenes addition to imidazolidines
Guarnieri-Ibáñez, A.; de Aguirre Fondevila, A.; Besnard, C.; Poblador Bahamonde, A. I.; Lacour, J.
Chem. Sci. 2021, 12, 1479-1485
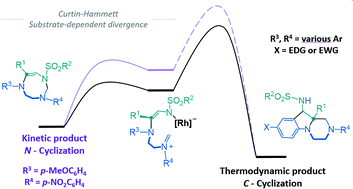
Hexahydropyrazinoindoles were prepared in a single step from N-sulfonyl triazoles and imidazolidines. Under dirhodium catalysis, α-imino carbenes were generated and formed nitrogen ylide intermediates that, after subsequent aminal opening, afforded the pyrazinoindoles predominantly via formal [1,2]-Stevens and tandem Friedel-Crafts cyclizations. Of mechanistic importance, a regiodivergent reactivity was engineered through the use of a specific unsymmetrically substituted imidazolidine that promoted the exclusive formation of 8-membered ring 1,3,6-triazocines. Based on DFT calculations, an original Curtin-Hammett-like situation was demonstrated for the mechanism. Further derivatizations led to functionalized tetrahydropyrazinoindoles in high yields.
For a highlight in the Swiss Science Concentrates of CHIMIA, see: https://doi.org/10.2533/chimia.2021.208
DOI : 10.1039/D0SC05725H
archive ouverte unige:148588
