The wings of a “genetic bird” protect us against viruses
UNIGE researchers have demonstrated that every population can protect itself against a broad range of viruses thanks to the two most diverse HLA immune genes in humans.
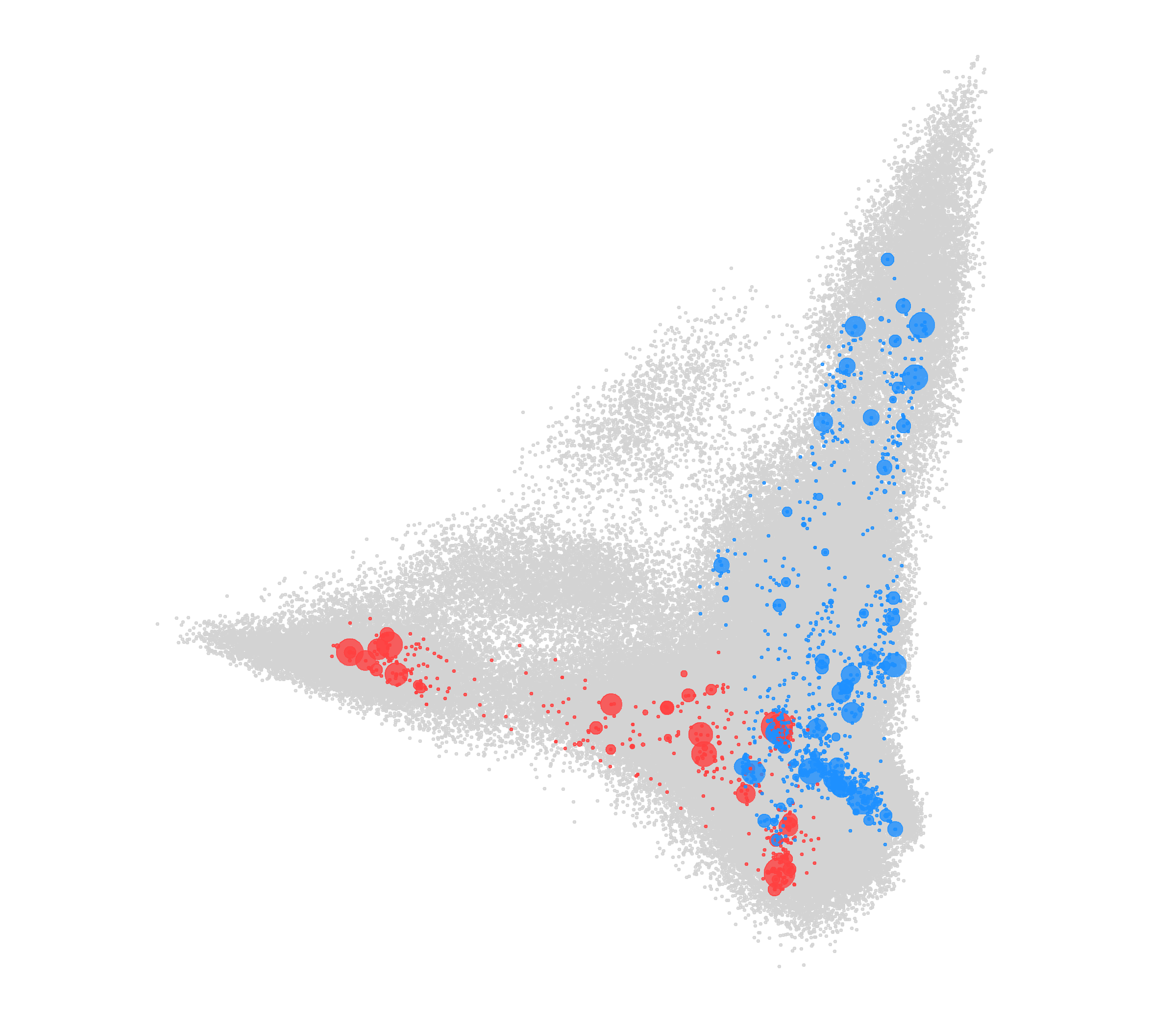
Modelling of HLA-peptide bindings forming the two wings of a bird in flight. © UNIGE
Do populations from different geographic regions have the same potential for defending themselves against pathogens and against viruses in particular? An analysis of human genomes, especially the HLA genes responsible for the so-called “adaptive” immune system, provide some possible answers to this question. These genes, which vary enormously between individuals, code for molecules capable of recognising the different viruses so they can trigger the appropriate immune response. In a study to be published in the journal Molecular Biology and Evolution, scientists from the University of Geneva (UNIGE), Switzerland, partnering with Cambridge University, identify the HLA variants that bind to families of viruses most effectively. The researchers show that, in spite of the great heterogeneity of HLA variants in individuals, all populations benefit from an equivalent potential when it comes to virus protection.
The human body’s first line of defence consists of recognising viruses as foreign bodies. Molecules known as human leukocyte antigens (HLAs) recognise the peptides – the small chains that make up a protein – in viruses. The HLA molecules then bind to these fragments and expose them on the surface of the cells, triggering a cascade of immune reactions designed to eliminate the virus.
The genes that code for HLA type A and type B molecules were of special interest to the researchers, since they play a primordial role in the ability to recognise the very wide range of different peptides derived from viruses that are pathogenic for humans. “These are the most polymorphic genes in our genome. We think that the existence of such a quantity of HLA genetic variants in humans is the result of natural selection, which – during our biological evolution – granted individuals better protection against the great heterogeneity of viruses. These genes enable us to constantly adapt our immunity,” explains Da Di, a researcher in the Department of Genetics and Evolution in UNIGE’s Faculty of Sciences, and the article’s first author.
Simulating immunity via computer modelling
“To simulate what happens when individuals are exposed to different viruses, we used several databases and computer tools to predict the binding strengths between HLA molecules and the peptides based on their physical and chemical properties,” reports Alicia Sanchez-Mazas, a professor in the Department of Genetics and Evolution, and the project’s leader. Two databases were used in the study: the first identified 3,000 different variants of HLA-A or HLA-B molecules. “We then created the second by randomly generating 200,000 peptides of nine amino acids – the building blocks that proteins consist of,” continues Dr Di. “This amazing number of peptides simulates the immense variety of pieces of virus possible in nature.” By modelling these binding forces, it was possible to observe that the HLA-A and HLA-B molecules distinctly recognise very different peptide families – and, it follows, potentially as many viruses. “When these binding forces are represented graphically, we can see that the peptides (grey in the figure) best recognised by the HLA-A molecules (red) form one wing of a bird; while those recognised by the HLA-B molecules (blue) form the other wing,” states José Manuel Nunes, a researcher in UNIGE’s Department of Genetics and Evolution, and co-author of the article. “Our immunity to the viruses, therefore, is like the two wings of a bird that play a joint and complementary role.”
When diversity means equality
The authors also investigated the variability of HLA molecules in terms of different populations, some of whom have a limited number of variants due to weaker genetic mixing. This suggests that certain populations would potentially be less well protected against some families of viruses. However, by analysing the variants of 123 global populations, the researchers found that each population systematically had molecules capable of recognising very different virus families. “This indicates that even in populations that have reduced genetic variability (such as native populations in Australia that have very few alleles for HLA genes), there are molecules in the immune system capable of countering viruses from very different families. This gives them a protective potential equivalent to other populations,” concludes Professor Sanchez-Mazas.
6 Jan 2021