INNOGAP - Proof-of-Principle Fund
Funded projects
Innogap funded projects in 2023
Monolithic silicon pixel sensor with a high time resolution
Dr. Thanushan Kugathasan and Prof. Giuseppe Iacobucci (DPNC) aim to build a monolithic silicon pixel sensor with a high time resolution of 10 picoseconds. The detector will be used for precise detection of photons in the visible and near-infrared spectrum. Advantages over existing solutions are an improved detection signal amplification with very low noise, higher acquisition rate and a uniform timing response with proper pixel design. Potential applications include high-precision LiDAR systems for autonomous vehicles, co-botics, satellite-to-satellite communication, and time-resolved Raman spectroscopy for studying rapid dynamic processes in materials and biology. The InnoGAP funding will be used to produce the detector and characterise it using the picosecond light source available at the university.
Prof. Giuseppe Iacobucci and Dr. Thanushan Kugathasan, Département de physique nucléaire et corpusculaire (DPNC), UNIGE

Projet LiteRev
Due to the volume of scientific articles published at unprecedented speeds, literature reviews have become slow, resource-intensive, and quickly outdated. Professor Keiser’s team has developed a new software LiteRev that aims at revolutionizing literature reviews by leveraging advanced technologies like natural language processing and machine learning. It enables users to streamline and automate the exploration of scientific articles across vast databases containing millions of open-access articles and preprints, offering fast and comprehensive insights into any research topic. LiteRev provides an overview of article topics and facilitates the prioritization of relevant content based on research inquiries. Researchers can engage with the engine to refine searches and receive targeted suggestions. In a real-world test, LiteRev already demonstrated a 56% reduction in workload compared to manual screening. The InnoGAP funding will be used to enhance the software's capabilities and performance by incorporating advanced features and functionalities, with the aim of refining LiteRev into the envisioned robust and versatile platform.

Establishing TGFbeta as a therapeutic drug
The laboratory of Pr Berhnard Wehrle-Haller has discovered an undescribed complex of the TGFβ family as therapeutic drug responding to a clinical unmet need.
Pr Bernard Wehrle-Haller and Dr Michaël Bachmann, Département de Physiologie Cellulaire et Métabolisme, University of Geneva, UNIGE.

Model 3D de clitoris et pénis stylisés basées sur des données in vivo
Dre Jasmine Abdulcadir et Dre Céline Brockmann ont réalisé un kit 3D démontable du trigone urogénital/ petit bassin, incluant le clitoris et le pénis, servant à l’enseignement et la clinique de l’anatomie et physiologie sexuelle et reproductive. Ce projet a montré un fort intérêt des spécialistes dans le domaine.
Dre Jasmine Abdulcadir, Département de gynécologie et obstétrique de la Faculté de médecine et médecin adjointe, service de gynécologie, HUG et Dre Céline Brockmann, adjointe et collaboratrice scientifique à la Faculté de médecine, co-directrice du Bioscope, UNIGE.

Protocole pour la détection de l’épilepsie
L’ équipe de la Pre Margitta Seeck a identifié des biomarqueurs de l’épilepsie, détectables automatiquement par électroencéphalographie (EEG). A terme, la technologie permettra de diagnostiquer de façon efficace l’épilepsie aux urgences et ce de manière automatisée et sûre. Les développements, financés au travers du subside INNOGAP, permettront d’améliorer, grâce notamment à l’intelligence artificielle, les performances de diagnostic avec des bénéfices évidents pour les patients.
Pre Magritta Seeck, Médecin adjointe agrégée responsable, unité d'exploration de l'épilepsie et EEG, Service de Neurologie, HUG.
Dr Eric Ménétré et Dr Stefano Gallotto, laboratoire de Neuropsycholinguistique, UNIGE, rattaché au laboratoire de cartographie cérébrale, Unité d’EEG et d’investigation de l’épilepsie, Département des Neurosciences cliniques, Service de Neurologie, HUG.

Genetic construct for selection of non-adherent cell lines
Gene therapy is transforming modern medicine by providing treatment and often even cure for diseases that were previously untreatable. For gene therapy, genetic information needs to be introduced into cells, and this is done with the help of so-called vectors. At present, the major limiting factor for gene therapy is the cost: the cure of a child with severe genetic immune deficiency (bubble child) costs ~1 million dollars. The cost for the cure of a patient with sickle cell anemia even exceeds 2 million dollars. A key factor for these high costs is the production of gene therapy vectors. Indeed, while gene therapy has shown major progress on most fronts, the production of the vectors remains virtually unchanged over the last 30 years.
The allover goal of the team of Dr. Maude Rolland and Pr Karl-Heinz Krause is the establishment of high quality gene therapy vectors at markedly reduced costs. For this task, several daunting biotechnology challenges need to be addressed. With the help of the Innogap grant, the team will address one of these challenges, namely the first step in the generation of cell lines that stably produce the gene therapy vectors by selecting cells that have incorporated high amounts of the DNA required to produce the vectors.
Dre Maude Rolland and Pr Karl-Heinz Krause, Département de pathologie et immunologie, UNIGE.

Fusion protein for the treatment of cancer
Development of a new approach to restore some anti-tumor immune response in HER2+ breast cancer patients.
Dr Aurélien Pommier, Immunopharmacology of Cancer, Section des Sciences Pharmaceutiques, UNIGE.
PROJETS FINANCÉS EN 2022
Non-polarizing optical module
We have developed an optical module that can replace a beam splitter or a dichroic mirror while preserving the polarization state of the transmitted and reflected beams.
If the performance is sufficient, this device could replace dichroic mirrors in standard multi-photon, fluorescence, Raman microscopy equipment and provide access to the valuable information carried by the polarization of light , which is usually degraded by the optics used in standard set ups .
Photo: From left to right are Dr Jérémie Teyssier and Dr Volodymyr Multian.
Inhibition of the WNT signalling pathway
We have identified chemical series called FSA with novel, specific and efficient inhibitory mechanism against a validated drug target pathway in cancers called Wnt. These compounds have anti-cancer effects in vitro and in vivo, with a marked decrease in adverse reactions associated with blunt Wnt inhibition typical for competitor compounds. With INNOGAP funding, we will continue lead optimization towards a second-generation, best-in-class Wnt inhibitors - an essential step towards clinical stage and commercialization of these results through creation of start-up with subsequent out-licensing to a big pharma company.
Photo: From left to right are Alexey Koval, Cédric Boudou and Gaël Greggio.
TESSA >> Planeto
Today the heating supply in buildings is more than 80 per cent fossil based. District heating and cooling networks (DHC) is an essential solution to reduce CO2 emissions in this sector. The adoption of the technology must quickly increase, limited mainly by the complication of designing DHC and the lack of adequate design tools. Our simulation software TESSA helps energy utilities and energy planners who want to design and develop new DHC projects by using machine learning algorithms to slash time, cost and accelerating the energy transition. Unlike traditional engineering consultancies approaches for energy planning tasks, we can produce results 10 times faster and provide geospatial modelling that is usually too time consuming and expensive to produce in early design phases. We aim to deliver a SaaS for DHC planning that allows our customers to generate the most important project KPIs (such as energy and CO2 savings, or investment cost).
With this INNOGAP, we aim to bring our software from a TRL 5 prototype to a TRL 7 SaaS, including implementation of IT best practices, and to develop a business model with our first customers, including defining the pricing model based on our add value and defining service offering for our spin-off Planeto.”
Photo: From left to right are PhD. Stefano Cozza, PhD. Jonathan Chambers, and Xiang Li.
PROJETS FINANCÉS EN 2021

Drugging the Epitranscriptome
Research in the Pillai lab is focused on understanding how RNA modifications are used for regulating gene expression. Using mouse models, the group has uncovered physiological functions of proteins that have the ability to ‘read’ RNA modifications. Use of protein biochemistry has allowed the group to identify functionally relevant protein complexes of reader proteins that are now going to be used for identifying small molecule disruptors that impede complex formation. The hope is that such inhibitors will be valuable for treating disease states caused by reader protein malfunction. The INNOGAP funding will be used for pilot studies towards this direction.
Photo: From left to right are Prof. Ramesh Pillai, Lingyun Li, Elena Delfino, Fabienne Fleury Olela and Pascal Gos.

SurvivAI (Dr Pierre Fabre)
This project targets slowly developing degenerative diseases, where affected cells or tissues take a long time to degenerate and where the testing of therapeutic interventions takes a lot of time. To address this time constraint, Dr. Pierre Fabre and Dr. Julien Prados have developed an artificial intelligence-based method to predict the long-term survival potential of single cells or small groups of cells. The AI is fed with single cell mRNA sequencing data and allows to predict survival scores for each cell or cell group. With the help of Quentin Lo Giudice, Nicolas Suarez and Silas Kieser, the project is about validating the model further. A patent has been filed on this technology.
Photo: upper row (left to right): Quentin Lo Giudice, Pierre Fabre et Silas Kieser; lower row: (left to right): Julien Prados et Nicolas Suarez.
FUNDED PROJECTS IN 2021
RefFIT
Optical experiments play an important role in many production and process development processes, in quality control and sorting applications in the materials industry. However, due to the complexity of the analysis of optical spectra, often valuable information contained in the spectra is not extracted or lost, especially in uniquely occurring situations. At the university of Geneva, a software called RefFIT was developed providing a no-code solution based on a library of scientific, interpretable models. With RefFIT, complex spectroscopic analysis becomes easy, fast and accessible for unskilled users. The INNOGAP funding will be used to develop a demonstrator for the photonics industry and to explore the broader potential of the analytical approach for other applications.
Photo: PhD Nicole Ruckstuhl and PhD Iris Crassee, Department of Quantum Matter Physics.
Targeting a link between metabolites and RNA to treat rare human diseases
The flow of genetic information held in our DNA passes through an RNA intermediate which is then used for producing proteins. After they have served this purpose, the RNAs are degraded by several decapping enzymes. The Pillai team is studying the molecular basis of rare human diseases caused by mutations in enzymes involved in RNA decay pathways. Using a mouse mutant for a human disease-related decapping enzyme, the Pillai team identified a link between accumulation of specific cellular metabolite and RNA decay pathways. The INNOGAP funding will be used to define this molecular pathway and explore therapeutic treatment options to target this aberrant pathway using the mouse model and patient-derived cell lines. Detection of this metabolite in body fluids as a diagnostic tool will be examined.
Photo: From left to right are Prof. Ramesh Pillai, Linyun Li, Michaela Dohnalkova, Elena Delfino, Pascal Gos.

Une solution pour produire des sphéroïdes cellulaires stables et standardisés
Le projet consiste en la création d'une start-up autour d'une nouvelle technologie pour la génération et la culture de sphéroïdes et organoïdes pour la recherche médicale et la thérapie cellulaire. En effet, dans le cadre des travaux de recherche sur la culture cellulaire 3D, Sanae El Harane a récemment développé la technologie 3D-AirLiWells (brevetée avec Unitec). Contrairement à la culture cellulaire traditionnelle en monocouche en 2D, la culture cellulaire en 3D est un environnement de culture qui permet aux cellules de se développer et d'interagir avec la matrice extracellulaire environnante en trois dimensions. Cette technologie a déjà été validée dans plusieurs applications montrant une haute standardisation et de nombreux avantages en comparaison à d’autres technologies de culture en 3D déjà sur le marché. Elle a été validée pour la première fois dans le cadre de la thérapie cellulaire pour la chirurgie reconstructive en utilisant des cellules souches de tissu adipeux. De plus, Sanae El Harane travaille actuellement sur l'optimisation d'un protocole de différenciation dopaminergique pour la thérapie cellulaire de la maladie de Parkinson. Enfin, dans le cadre de collaborations, elle a également permis le développement d'organoïdes pour modéliser une pathologie du foie et le cancer du sein.
Actuellement, la technologie a été validée dans un format de plaque 6 puits. Le fond Innogap permettra de développer les 3D-AirLiwells vers des formats plus petits comme les plaques 24 ou 96 puits qui permettront ainsi du criblage à haut débit. Le fond va également permettre d’élargir les applications à travers un test interne de la technologie au sein de la faculté de médecine de Genève. De plus, il permettra d’optimiser davantage le produit grâce aux différents retours.
Photo: Sanae El Harane

Novel screening method/model for auditory neurogenesis
The intrinsic limited stemness of mammalian sensory progenitor cells has always been an important limitation for in vitro investigations and further development of therapies as well as regenerative medicine for sensory disorders. The present invention relates to reprogramming methods for inducing stemness into sensory neuroprogenitor (ANPG) cells and uses of ANPG cells, or cells having similar properties to ANPG cells. Stemness-induced (reprogrammed) ANPG exhibit robust intrinsic self-renewal properties (beyond 40 passages) and at any passage, differentiate into functional sensory neurons. This invention does not only provide a unique tool to understand ANPG self-renewal and regeneration in mammals, but also delivers a robust in vitro platform for high-throughput screening assays and further therapeutics development. A further advantage of the present invention is the great reduction in animal numbers needed with major implications in 3R efforts to replace, reduce and refine the use of animals in research and therapeutic settings, also reducing associated costs and administrative and ethical burden. The present cell line and reprogramming methods offer great possibilities for high throughput in vitro investigations as well as regenerative medicine for researchers and biotech companies in the field of sensory neurosciences.
Photo: de gauche à droite Prof. Pascal Senn (professeur associé), Mrs Rebecca Sipione (PhD student) ; Dr Francis Rousset (PhD, Maitre Assistant), Mr German Nacher-Soler (PhD student). L'image au fond montre des neurones auditifs matures de souris générés à partir de progéniteurs régénérés par reprogrammation.

Synthesis and biological evaluation of small fragment inhibitors of TNFR1
Deregulation of the Tumor Necrosis Factor (TNF) pathway is responsible for the pathological onset of various autoimmune disorders and the perpetuation of chronic inflammation. The current standard of care for a broad spectrum of inflammatory conditions, such as rheumatoid arthritis, Crohn's disease, and psoriasis, consists of using TNF inhibitors that directly prevent TNFα binding to its two receptors: TNFR1 and TNFR2. However, total TNF inhibition often leads to severe side effects such as an increased risk of developing multiple sclerosis and the reactivation of tuberculosis, mainly caused by the impairment of TNFR2-mediated response.
Hence, by combining fragment-based in vitro screening, mutational studies, and iterative molecular modeling, we have identified preliminary hits that selectively block TNFR1 pro-inflammatory activity. The Innogap fund will help acquire deep insights into the MoA (mechanism of action) of these scout molecules and refine a three-dimensional model of the receptor for developing analogs with enhanced inhibitory activity towards TNFR1.
Photo: from left to right Prof. Dr. Leonardo Scapozza, Sara Pannilunghi
FUNDED PROJECTS IN 2020
Team Tracker: une nouvelle technologie qui vient en aide au secteur médical ainsi qu'à d'autres secteurs
Les erreurs médicales sont l'une des causes majeures de décès dans le monde et nécessitent donc une attention spéciale. La technologie « TeamTracker » vise à aider les praticiens à améliorer leurs capacités de travail en équipe, de coopération et vise à minimiser leur risque de commettre des erreurs. Il s'agit d'un logiciel d'analyse automatique de la performance individuelle et collective permettant de monitorer une activité donnée à l'aide d'indicateurs de performances intelligents adaptés à l'activité du secteur concerné. Le logiciel dispose d'une interface de visualisation 3D capable d'enregistrer et de rejouer les données relatives aux mouvements et à la voix (entrées du système) acquises au cours d'une session. Cela permet par la suite, entre autres, à l'examinateur, d'avoir accès à un rapport automatique contenant des indicateurs de performance des participants lors d'une séance d'évaluation et ainsi avoir une mesure quantitative et non-biaisée de celle-ci. Le financement INNOGAP sera utilisé pour la validation du logiciel et pour continuer son développement qui vise, entre autres, à enrichir la palette d'indicateurs existantes, à perfectionner l'algorithme et d'implémenter une intelligence artificielle de type Deep Learning pour détecter les profils performants et/ou à risque dans une équipe.
Photo: Dr. Donald Glowinski (gauche) et M. Emmanuel Badier (droite). En partenariat avec le Centre Interprofessional de Simulation, Genève.

Detection of cells circulating in the whole blood
This multidisciplinary project (Applied physics – UNIGE, Neurosciences – UNIGE, and Tissue engineering lab – HEPIA) aims to develop a device for the detection of individual cells circulating in the whole blood. The technology relies on a cost-effective laser originally developed for telecom application and has implications for the bio-research and medical communities. The high-sensitivity/selectivity detection is based on a novel mechanism that avoids blood autofluorescence problems. One key application is to trace cells in the circulatory system during regenerative medicine and immune therapies.
Photo (left to right): PhD. Adrien Roux, PhD. Gabriel Campargue, PhD. Marisa Jaconi Dévaud and PhD. Luigi Bonacina.
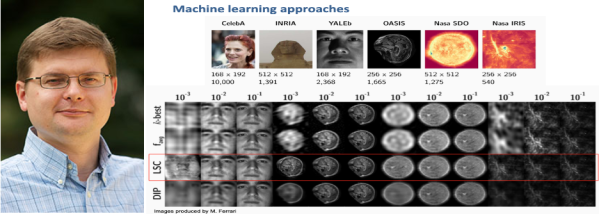
L'échantillonnage intelligent avec la reconstruction par apprentissage automatique
L'imagerie en général est gourmande en données de stockage au moment de la formation de l'image. L'invention du Prof. Slava Voloshynovskiy et de ses collègues vient en aide à ce problème car elle permet d'économiser des ressources de stockage lorsque l'image est formée et de traitement de données avec une amélioration de plusieurs ordres de grandeur. La méthode consiste à traiter l'image non pas dans leur ensemble mais au moyen d'un échantillonnage intelligent car la machine a préalablement appris à reconnaître le type d'image à acquérir. La technologie est particulièrement utile dans les domaines de l'imagerie médicale, l'imagerie hyperspectrale ou encore dans l'astronomie. Le financement INNOGAP sera utilisé pour faire développer une version robuste avec une interface agréable afin de pouvoir faire des démonstrations auprès des sociétés ou des investisseurs intéressés.
Photo: Prof. Slava Voloshynovskiy et une image comparative de son invention LSC.

A molecule with high potential therapeutic activity against Sars-CoV-2
Prof. Matile and Dr. Sakai have identified a small molecule with potential therapeutic activity against Sars-CoV-2 using a pseudoviral entry assay. Preliminary results showed that one small molecule demonstrated an IC50 of 50 µM in the absence of cytotoxicity. Positive feedback has already been received from Roche, MSD, Merck and Novartis. INNOGAP funding will be used to test analogous chemicals that may have higher potency and to further understand their mechanism of action.
Photo: Prof. Stefan Matile, Department of Organic Chemistry, University of Geneva.
A promising cell-based assay in the fight against Sars-CoV-2
In the context of dealing with the COVID-19 pandemic, Prof Krause and his team have developed a cell-based, high-throughput screening assay that may be used to indicate whether a person is (still) immunized against the Sars-CoV-2 virus. This assay may also be used as a screening assay for anti-COVID-19 drugs. INNOGAP funding will be used to further optimize this assay and to continue further validation using the sera from large numbers of infected and non-infected patients.
Photo (left to right): Mr. Sébastien Mosser, Mr. Fabien Abdul, Mr. Olivier Preynat-Seauve, Ms. Aurélie Caillon and Prof. Karl-Heinz Krause.

A device for the treatment of acute ischemic stroke (AIS)
AIS is the first cause of acquired neurological deficit and the third cause of death in the adult population in the Western world. It is due to the occlusion of a brain vessel by, for example, a clot. Since 2015, the removal of the clot is done using the method of mechanical thrombectomy. Prof. Paola Machi and his collaborators developed a prototype of medical device which would help them recognize different occlusion patterns and characteristics by means of impedance sensors present in the instrument. The INNOGAP funding will be used to purchase human vascular training flow models and interventional tools such as micro-guidewires and micro-catheters.
Photo (left to right): Olivier Brina, Paolo Machi and Philippe Reymond.

A novel method to produce vesicular nanocarriers
Nanomedicines have an increasingly wide application in the prevention, diagnosis, and treatment of diseases. In order to produce these vesicular nanocarriers, Dr. Darade and Prof. Kalia have developed an improved method which uses cryo-milling techniques. These should enable scale-up production of nanocarriers based on a solvent free approach that has a shorter processing time and potential advantages for bulk storage. INNOGAP funds will be used to optimize and validate the process, to characterize the stability of the formulation and to compare the efficacy of nano-encapsulated medicines prepared by conventional methods and by the method described here.
Photo (left to right): Prof. Yogeshvar Kalia, Mr. Aditya Darade and PhD. Maria Lapteva.
A novel antibacterial and antiviral nanocoating
Spread of pathogenic microbes and antibiotic-resistant bacteria in healthcare facilities and public space is a serious public health challenge. In order to tackle the problem, Mikhail Kryuchkov, a post-doc researcher at the laboratory of Prof. V. Katanaev, developed a method for producing a cicada wing surface-like nanocoating covered by nanoparticles, which is applicable on all large-scale surfaces, cost effective and stable for at least one-week period. The INNOGAP funding will be used to tets different nanoparticles and to validate the technology according to the ISO requirements.
Photo: PhD. Mikhail Kryuchkov, Department of Cell Physiology and Metabolism, University of Geneva.
A ring-shaped device to assist surgeons during brain tumor removal surgical procedures
Dr. Zaccaria and Prof. Momjian have developed a single use medical device which will help surgeons during brain tumor removal surgical procedures. The invention consists of an MRI-compatible ring that will give the neurosurgeon the 3D-position of tumoral regions in the patient’s cerebral parenchyma. This should reduce overall medical costs by reducing the occurrence of second/redo surgeries and will help in training less experienced surgeons. The inventors will use the INNOGAP fund to produce a prototype, to test it and to validate the technology in a representative environment.
Photo (left to right): PhD. Shahan Momjian and PhD. Affif Zaccaria.

Characterization and assessment of a new glycosylation system
Modification of proteins by glycosylation is fundamental to many biological processes and valuable for the development of therapeutic proteins. Prof. Patrick Viollier identified and characterized a protein from Caulobacter crescentus, a new glycosyltransferase, capable of glycosylating proteins using sialic acid and pseudaminic acid. He also engineered a new strain which represents the first in vivo bacterial system to glycosylate soluble proteins. The INNOGAP funding will be used to test the new-engineered bacterial strain for glycosylating human proteins.
Photo (left to right): PhD. Nicolas Kint and PhD. Patrick Viollier.
FUNDED PROJECTS IN 2019
SPRING 2019
Method for object recognition and/or verification on portable devices (Prof. S. Voloshynovskyy)
Le projet du Prof. Voloshynovskyy a pour objectif de développer une application mobile permettant d’identifier et de vérifier l’authenticité de descripteurs extraits des surfaces de produits et/ ou de leurs emballages à la sortie de leur production. L’objectif est que des consommateurs ou des inspecteurs puissent, au moyen de la caméra de leur téléphone portable, vérifier l’authenticité de produits disponibles sur le marché ; des médicaments par exemple. La technologie est protégée par une famille de brevets.
Micro Magnet (Dr. Alexey Kuzmenko; M. Adrien Bercher; Dr. Jean-Marie Poumirol)
The aim of this Innogap project is to build an ultra-small (few mm) and ultra-strong (1.6 T) magnet for scanning local-probe measurement, like STM, AFM or s-SNOM. The special designed geometry offers multiples avantages among them no stray field outside the magnet and an easy access to the maximum field area from the top.
Phospho-prodrugs of 5-aminolevulinic acid (Prof. Norbert Lange)
Ce projet a pour but de faire la preuve de concept chez l’animal d’une nouvelle génération d’agents de contraste dérivés de l’acide aminolévulinique ALA en administration systémique. Le produit est destiné à l’imagerie par fluorescence des tumeurs et permettre une résection optimale de la masse tumorale. La première indication est le cancer du sein, pour lequel il n’existe actuellement pas de diagnostic par imagerie fiable. (700'000 nouveaux cas de cancer du sein par année EU et US).

Détection précoce de l’infection de cultures agricoles, spatialement résolues en temps réel (Dr. Vasyl Kilin, Prof. Jérôme Kasparian and Prof. Jean-Pierre Wolf)
The project of the team of Prof. Wolf deals with the use of pesticides in crop, and in particular in vineyards. By detecting in real-time and with a high spatial accuracy the presence of spores like mildew, this will allow the vine makers to treat very gently their grapes. The system developed by the team combines laser technology and AI to recognise the spore in air.
Production of 3D-printed shell for breast radiotherapy (Dr. G. Dipasquale)
In radiotherapy, high intensity X-ray are used to destroy the tumor while trying to minimize the damage to surrounding healthy tissue. During treatment, it is crucial to well immobilize patients, as the process requires multiple repositioning sessions. Ms. Giovanna Dipasquale has developed a new method to produce personalized breast support (shell) created by 3D-printing using surface scanner images of patients. The Innogap funding will be used to test the benefit of this innovation in a clinical study.
Utilisation de la technologie des tetramers dans le diagnostic et dans la thérapie du syndrome des antiphospholipides - SAPL (Dr. K Brandt)
Le syndrome des antiphospholipides (SAPL) est une maladie auto-immune causant des thromboses et/ou des complications de grossesse, comme par exemple des fausses couches répétées. La détection du SAPL est très compliquée, car les causes et les manifestations du SAPL sont variables selon les patients. Par conséquent, les tests diagnostiques actuels se basent sur la présence de symptômes cliniques et la détection de plusieurs marqueurs à au moins 2 moments différents. Dr. Karim Brandt a identifié un motif commun reconnu par tous les auto-anticorps SAPL et a développé certains peptides permettant de diagnostiquer la présence d’auto-anticorps SAPL. Le financement Innogap servira à prototyper un test diagnostique du SAPL basé sur ces peptides.
Bacterial nanocellulose as a matrix for a new generation wound dressing (Dr. François Barja)
Dr. François Barja generated a genetically engineered bacterium to increase the production of biocellulose. In collaboration with the CHUV, he has investigated the properties of biocellulose for wound dressing for burn victims and chronic injuries.
Innogap funding will allow the construction of a bioreactor to standardize, optimize and control the physicochemical parameters needed in the production of biocellulose as a component of a medical device.
FUNDED PROJECTS IN 2018
FALL 2018

Développement d’un nouvel analgésique sélectif pour le traitement des douleurs neuropathiques chroniques
Le groupe du Prof. Jules Desmeules a démontré que le métabolite actif d’un traitement antiépileptique, le Chobazam possède une activité sélective sur les récepteurs GABA pour traiter les douleurs chroniques sans avoir d’effets sédatifs.
Une première étude clinique de phase Ib a été réalisée aux HUG. Le financement Innogap permettra de libérer un nouveau batch GMP certifié du métabolite pour démarrer une étude clinique de phase IIa

Création d’une éponge cellulaire régénératrice et bio-résorbable pour le traitement des plaies graves
Le Dr. Olivier Preyna-Seauve du laboratoire de thérapie cellulaire expérimental en collaboration avec le Dr. Nicolò Costantino Brembilla ont créé un nouveau pansement biologique destiné à produire de façon durable des facteurs cicatrisant et vascularisant.
Le financement Innogap permettra de valider l’efficacité de ce pansement sur un model animal.
SPRING 2018

Production de technologie recombinante (Dr W. Du Fresne Von Hohenesche, P. Hammel et Prof. P. Cosson)
Les anticorps monoclonaux font partie des molécules les plus prometteuses dans les domaines thérapeutiques et diagnostiques. En 2014, le Prof. Pierre Cosson a mis sur place une nouvelle plateforme d’anticorps recombinants à la faculté de médecine pour des projets de recherche académique.
Le financement de 30'000 CHF a pour objectif d’améliorer et d’optimiser la production de ces anticorps

Inhibition de l’assemblage d’une matrice de fibromectine par interférence de récepteurs intégrins alpha5 beta1 (Prof. B. Wehrle-Haller)
Les récepteurs intégrins sont reconnus pour être des cibles thérapeutiques pour traiter différentes pathologies et notamment le cancer. Le laboratoire du Prof. Bernard Wehrle-Haller a démontré qu’une nouvelle protéine de 60 acides se liait avec le récepteur integrin alpha5-beta1 et inhibait la formation de fibronectin pour empêcher la croissance des tumeurs. Les analyses de structure 3D indiquent que la protéine pourrait avoir une meilleure interaction avec le récepteur qu’un anticorps (Volociximab) utilisé pour une étude clinique et qui a été récemment abandonnée pour un manque d’efficacité.
Le financement de 30'000 CHF va permettre de faire produire cette protéine en quantité suffisante pour tester sa spécificité avec le récepteur alpha5-beta1 et déterminer son degré d’immunogénicité
Nouveau thérapeutique contre l'action de toxines et les infections virales (Dr T. Hannich, Dr A. Galih et Prof. H. Riezman)
Le groupe de Howard Riezman (Faculté des Sciences/NCCR Chemical Biology), en collaboration avec les groupes de Jean Gruenberg (Faculté des Sciences) et Gisou van der Goot (EPFL), a découvert une molécule qui inhibe les effets néfastes de certaines toxines, dont celle d'anthrax, et la propagation de nombreux virus. Le financement Innogap permettra d'optimiser la structure de cette molécule et de confirmer son effet sur des virus et toxines dangereux pour l'Homme.

Projet de découverte de médicaments (V. Jaquet, Y-A Cambet, F. Augsburger, Prof. K-H Krause)
Le groupe de Vincent Jaquet a utilisé la plateforme de criblage READS de la Faculté de Médecine pour identifier plusieurs petites molécules qui inhibent une cible moléculaire liée à la gestion des radicaux libres. Le financement Innogap permettra d'améliorer les propriétés chimiques des composés les plus prometteurs et d'effectuer certaines études précliniques requises pour démarrer un projet de développement de médicaments pour des indications variées allant de l'hypertension aux complications vasculaires liées au diabète.

New design of micro RNAs for gene therapy vectors (Dr M. Alessandrini)
This project is based on a new vector able to very effectively suppress the expression of certain genes. Whilst this vector was initially developed to suppress the expression of CCR5 in CAR-T cells to prevent HIV infections, this new project aims at diversifying the applications, for example to fight solid tumors.
FUNDED PROJECTS IN 2017
FALL 2017

Novel pathway to improve cancer immunotherapy (Prof. Walter Reith, Dr. Thibaut De Smedt and Isabelle Dunand-Sauthier - not on picture)
The immune system plays fundamental roles in suppressing the initiation of malignant neoplasms, inhibiting tumour progression and promoting tumor elimination. Newly developed CAR-T cell therapies largely rely on this natural phenomenon. However, solid tumours use various mechanisms to escape immune surveillance, for example by depleting their microenvironment of metabolites that are essential for anti-tumoral function of immune cells.
The group of Prof. Walter Reith has developed a method to enhance CAR-T cell therapy by avoiding the above evasion mechanism in the micro-environment of solid tumours. The Innogap funding will be used to confirm effectiveness of the method in mice and in human CAR-T cells.
A2AR NAMs for cancer immunotherapy (Prof. Leonardo Scapozza)
Lack of oxygen in the center of solid tumours is known to induce elevated extracellular adenosine which signals through A2AR receptors resulting in profound immunosuppression, and thus tumour evasion from immune surveillance.
The group of Leonardo Scapozza has identified three hit series of negative allosteric modulators of the drug target A2AR that display ideal characteristics as potential anti-cancer drug candidates. The Innogap project aims at advancing the chemical development of these hit series, as well as partial biological validation of the compounds in translational assays relevant for cancer immunotherapy.
Development of a new Elisa assay for a CatK-digest Periostin fragment (Prof. Serge Ferrari)
Antibacterial dressing for improved would care (Prof. Gerrit Borchard, Dr Olivier Jordan, Dr Viorca Patrulea)
Monolithic detector (Prof. Giuseppe Iacobucci, Dr. Lorenzo Paolozzi)
The aim of this project is to develop an ionizing particle detector, based on the time-of-flight technique (TOF), integrated in a commercial CMOS process and with characteristics such as a temporal resolution of about ten picoseconds and a very low power consumption.
In addition to positron emission tomography (PET) measurements, this invention could easily find its place in devices using light imaging, detection and ranging (LiDAR) technology, which is increasingly found today in autonomous cars, drones, TOF cameras, smartphones, etc where the importance of fast and accurate measurements and miniaturization are more and more required.
SPRING 2017

Harmonic Nanoprobes for biomedical applications (Prof. Jean-Pierre Wolf, Dr. Vasyl Kilin, Dr. Luigi Bonacina)
- spectrally harmonics are generated hundreds of nanometers away from excitation
- photoimmortality
- unlimited number of emitted photons
- spectral flexibility of excitation and emission
- determination of individual probe spatial orientation
- phase-coherent optical response
- possibility to use the same probes in visible and IR region.
Conjugate-based micelle formulations and their use for drug delivery (Prof. Y. Kalia and S. Kandekar)
Acne vulgaris (acne) is a highly prevalent dermatological condition of the pilosebaceous unit (PSU). lt affects a significant proportion of the population - although not life threatening, if left untreated, it can have serious physical and psychological consequences. The actual treatment has many side effects that can affect patient compliance. This Innogap project aims at improving the actual acne treatment by developing a drug that will target specifically the PSU.

Nouvel agent de contraste pour visualiser le tissu adipeux brun par CT (Prof. E. Allémann, L. Vinet et A. Babi)
La visualisation du tissu adipeux brun se fait actuellement par 18F-déoxyglucose-PET, une technique peu précise et influencée par des facteurs métaboliques. Ce projet INNOGAP vise à optimiser un nouvel agent de contraste qui permet d’une part une visualisation du tissu adipeux brun par tomographie assistée par ordinateur (CT) - technique plus répandue dans les hôpitaux que le PET - et d’autre part une administration orale, avec des doses d’irradiation plus faibles. Ce nouvel agent de contraste sera utile pour la recherche et le développement de médicaments, ou la stratification de patients et le monitoring thérapeutique dans les domaines de l’obésité et le diabète, la « brunification » du tissu adipeux blanc étant actuellement une piste thérapeutique très active.
FUNDED PROJECTS IN 2016
FALL 2016

MIAPARLE: Méthode Interactive d'Aide à la Prononciation pour l'AppRentissage d'une Langue Etrangère: aspects prosodiques (Dr. S. Schwab, J.-P. Goldman).
MIAPARLE est une application web qui permettra d'améliorer son accent dans une langue étrangère telle que l'anglais, l'espagnol, l'allemand ou l'italien. Centrée sur l'accentuation et l'intonation, l'application consistera en une série d'exercices alliant informations auditives et visuelles pour entraîner l'apprenant à percevoir et à prononcer correctement les syllabes accentuées (c'est-à-dire l'accent tonique) dans une langue étrangère. En effet, les syllabes accentuées, importantes pour la compréhension et la production orale dans une langue étrangère, sont prononcées avec une augmentation de la longueur, de la hauteur et de l'intensité.
Le logiciel, qui sera développé avec une enveloppe de CHF 30'000, reflètera une approche innovante, l'enseignement de l'accentuation étant peu traité dans les méthodes traditionnelles existantes qui se concentrent surtout sur la prononciation des sons.
Méthode de modulation post-transcriptionnelle de l’expression génétique et de synchronisation cellulaire pour sensibiliser les cellules tumorales aux thérapies ciblées (Dr Rastine Mérat)
Drug permeability enhancer (Prof. G. Borchard, S. Ragupathy)
Anti-cancer compounds often do not reach the center of a solid tumour because cell-cell junctions between tumour cells inhibit tissue penetration. Similarly, transmucosal drug delivery and mucosal vaccination is often not very efficient, as epithelial cell-cell junctions hinder the drug permeability. In both cases, a transient permeabilisation of the tissue would help the therapeutic agent or the vaccine penetrate into the tissue to exert their action.
This INNOGAP project aims at testing new peptides (invention) in cancer 3D (spheroïd) cell models to confirm the enhancement of tissue penetration resulting from the increased paracellular flux. Funding : CHF 30’000.
SPRING 2016

Improved image compression taking quantum effects into account ( Prof. Hugo Zbinden , Dr. Bruno Sanguinetti, Dr. Anthony Martin ).
This patented technology allows to compress images by a factor 10 without compromise on image quality (or very little). The CHF 30’000 INNOGAP subsidy aims at allowing the inventors to produce a prototype which will enable them to make demonstrations in front of potential licensees.

Logiciel de commande d’images à distance en salle d’opération : KiOP ( Prof. Christian Lovis , Dr. Victor Dubois-Ferrière, Thomas Mikael Strgar ).
Le logiciel KiOP développé par les HUG et l’HEPIA fait l’interface entre une caméra à reconnaissance de mouvement et un logiciel d’imagerie médicale et traduit les gestes du chirurgien captés par la caméra en action : zoom, scroll, contraste, etc. Le subside INNOGAP de CHF 30'000 permettra de tester en situation réelle aux HUG la version 2.0 du logiciel qui fonctionne avec la caméra Kinect 2 et d’optimiser ses fonctionnalités.
One-step synthesis of functionalized PLGA/PLA/PCL (Prof. E. Allémann, P. Maudens, C. Thauvin)
Prof. Allémann’s team developed a rapid and easy method to functionalize a polymer family currently used for drug delivery applications. Improvements over the state of the art impact for example (i) controlled release, (ii) drug loading levels, (iii) biocompatibility, (iv) targeted delivery (v) fluorescence imaging, etc. The project aims at producing a proof-of-concept, by testing several classes of polymers produced with the method.
FUNDED PROJECTS IN 2015
FALL 2015

BTN2A2 inhibitors as cancer immunotherapeutics ( Prof. Walter Reith ).

Des chaussures intelligentes pour les diabétiques ( Prof. Zoltan Pataky ).
Les ulcères du pied diabétique sont causés par une combinaison de neuropathie périphérique et répartition anormale de la pression plantaire. Les ulcères des pieds sont une complication fréquente du diabète pouvant mener à l’amputation dans les cas sévères. Pour aider les patients diabétiques à décharger les zones de haute pression plantaire afin d’éviter, ou favoriser la guérison, des ulcères plantaires, Dr. Zoltan Pataky du Service d’enseignement thérapeutique des maladies chroniques des HUG, en collaboration avec le groupe du Prof. Yves Perriard du Laboratoire des Actionneurs Intégrés de l’EPFL, ont développé une semelle intelligente à insérer dans les chaussures des diabétiques qui mesure et ajuste automatiquement la pression sous tout le pied. Le système de mini-valves permettant ce réglage a été breveté, et un prototype d’une valve a été testé. Le financement Innogap permettra aux inventeurs de produire et tester un prototype complet de semelle comprenant une cinquantaine de mini-valves pour couvrir la totalité de la plante du pied. Ce projet a fait l’objet d’un article dans Le Temps. Photo: Prof. Pataky.
SPRING 2015

Molécules de plantes pour traiter les maladies parasitaires ( Prof. Jean-Luc Wolfender ).
Le laboratoire de Phytochimie du Prof. Jean-Luc Wolfender collabore avec l’Université de Sao Paulo pour isoler des nouveaux composés chimiques à partir de plantes brésiliennes afin de traiter des maladies parasitaires comme le paludisme, la maladie de Chagas et la Leishmaniose. Les extraits obtenus ont permis de caractériser trois nouveaux composés qui appartiennent à une classe chimique très rare de biflavonoides. Des études in vitro et in vivo ont montré que les composés isolés possédaient une activité remarquable contre le trypanosoma cuzi, le parasite responsable de la maladie de Chagas. Le financement Innogap permettra d’effectuer des études de toxicologie dont les protocoles et les résultats sont reconnus par les industries pharmaceutiques. Photo (de gauche à droite): Prof. Ferreira-Queiroz, Prof. Wolfender.

EMOTEO: une application smartphone pour la gestion des émotions ( Dr Nader Perroud ).
Le service des spécialités psychiatriques des HUG a développé une nouvelle application smartphone ayant pour objectif d’aider des personnes souffrant d’un trouble de personnalité borderline qui sont victimes de crises émotionnelles. L’application utilisée comme un outil psychoéducatif possède une échelle d’évaluation de la tension interne et des exercices pour faire baisser la tension des patients et prévenir ainsi des comportements suicidaires. Le financement Innogap a pour objectif d’améliorer le graphisme de l’application, d’augmenter le nombre d’exercices et de faire une version pour Android. Photo (de gauche à droite): Dr Perroud, Dr Prada, M. Bouillault.

A color-changing ink that is DNA-operated ( Prof. Nicolas Winssinger ).
Dans le sillage des récents scandales alimentaires, le Dr. Gianpaolo Rando et le Prof. Nicolas Winssinger ont développé une méthode de détection d’aliments qui peut être pratiquée par le consommateur, en-dehors d’un laboratoire et sans séquençage onéreux. Une fois mis au point, le test sera sous forme de bandelettes de papier pré-imprimées et permettra de détecter la présence de certains aliments donnés (comme par exemple du porc ou du cheval), voire de certains pathogènes contaminants communs, à l’œil nu ou avec une application tournant sur smartphone. Le financement Innogap permettra de confirmer que les colorants utilisés pour la détection peuvent être fixés sur la bandelette de papier et de tester la sensibilité de la méthode. Photo (de gauche à droite): Prof. Rando, Prof. Winssinger.


babeIDr ( Prof. Pierrette Bouillon , Prof. Jean-Michel Gaspoz, Dr. Herve Spechbach, Dr Sophie Durieux-Paillard et Patricia Hudelson) .
Le Prof. Pierrette Bouillon travaille depuis de nombreuses années sur des technologies de reconnaissance vocale basées sur des grammaires. BabelDr est une application smartphone pour les services d’urgences hospitalières; elle permettra de diminuer les erreurs de diagnostic dues aux problèmes de langues. Aux HUG, 52% des patients admis aux urgences sont étrangers et 10% ne parlent pas du tout le français. Le prototype de BabelDr sera disponible en trois langues: espagnol, tigrignia et arabe. Il comprendra trois diagnostics courants: migraine, douleur de poitrine et douleurs abdominales. Photo de haut (de gauche à droite): Prof. Bouillon, M. Tsourakis, M. Rayner (FTI). Photo de bas (de gauche à droite): Dr Spechbach, Dr Durieux-Paillard, Dr Hudelson (HUG).

nano-R2OBBIE 3D ( Prof. Michel Milinkovitch ).
Le Professeur Michel Milinkovitch, au département de Génétique et Evolution, a développé un bras robotisé rapide «R2OBBIE 3D» muni d’une source de lumière et d’une caméra. Il permet de scanner très finement des objets en 3D et de les associer à des images haute résolution. Cette technique est utilisée notamment pour l’étude de la microstructure de modèles 3D tels que lézards, serpents etc. D’autres applications plus industrielles sont aussi possibles. Afin d’améliorer encore la résolution de R2OBBIE et de passer de l’échelle du micron (10^-6 m) à celle du nanomètre (10^-9 m) le Prof. Milinkovitch propose d’utiliser une source de lumière UV. Ceci nécessite des adaptations scientifiques et techniques importantes et fait l’objet de cette demande INNOGAP. A l’issue de ce projet, de nouveaux domaines d’applications devraient s’ouvrir tels que les applications dans le domaine de la police scientifique. Photo (de gauche à droite): M. Martins, Prof. Milinkovitch.
FUNDED PROJECTS IN 2014
FALL 2014
CPR Testlung: un poumon pour l'arrêt cardiaque ( Dr Georges Savoldelli ).
Le Dr Georges Savoldelli responsable du centre SIMULHUG et le groupe du Prof. Jean-Christophe Richard ont imaginé un poumon test novateur pour l’arrêt cardiaque pouvant être intégré dans un mannequin de type Ambu. Le financement Innogap permettra la fabrication d’un prototype fonctionnel. Cette innovation sera principalement un outil pour la formation et l’enseignement pour juger de la qualité d’un massage cardiaque et étudier l’influence des compressions thoraciques sur la mécanique respiratoire et sur l’hémodynamique.

Active Content Fingerprinting ( Professeur Svyatoslav Voloshynovskyy ).
Le projet du Prof. Voloshynovskyy, au département d'informatique vise à protéger des objets contre la contrefaçon. La technologie brevetée protège notamment le fait de prendre une photo d'une région d'un emballage à la sortie de l'usine, de transformer digitalement cette photo et d'enregistrer le résultat dans une base de données. Pour vérifier l'autenticité d'un produit, on le photographie dans une région définie de l'emballage et grace à un logiciel, on compare l'image avec le contenu de la base de données. Cette technique très robuste et rapide permettra aux industries l'utilisant, d'une part d'économiser sur l'impression de signes distinctifs uniques et d'autre part de plus efficacement identifier les fabricants et distributeurs de contre-façons. Le subside INNOGAP permettra de réaliser le premier prototype entièrement opérationnel de la technologie.
SPRING 2014

Une nouvelle méthode pour analyser des récepteurs olfactifs ( Professeurs Ivan Rodriguez et Alan Carleton ).
Les groupes d’Ivan Rodriguez et d’Alan Carleton s’intéressent aux récepteurs olfactifs exprimés par des neurones sensoriels. Les humains possèdent environ 400 récepteurs olfactifs différents qui seuls ou en combinaison avec d’autres récepteurs, répondent aux divers odorants auxquels ils sont exposés. L’étude de ces récepteurs olfactifs est techniquement ardue et de ce fait, à ce jour, très peu de paires odorant-récepteur(s) ont été identifiées. Les chercheurs ont développé une nouvelle méthode pour analyser quels récepteurs répondent à un odorant donné. Comme la méthode a été initialement développée chez la souris, le financement Innogap permettra aux chercheurs de confirmer que la méthode est aussi applicable pour analyser le répertoire olfactif humain.

Une nouvelle méthodologie pour le traitement par chémo-embolisation du cancer du foie ( Professeur Gerrit Borchard ).
Le cancer du foie est actuellement souvent traité par chémo-embolisation, soit l’injection d’agents embolisants dans une artère qui alimente la tumeur. Le traitement comporte en général d’une part des particules embolisantes (pour interrompre l’irrigation de la tumeur) et d’autre part un agent de chimiothérapie (pour tuer les cellules cancéreuses). Comme beaucoup d’agents de chimiothérapie sont très hydrophobes et les particules embolisantes sont souvent hydrophiles, il est actuellement difficile d’administrer les 2 types d’agents en même temps, voire de manière synergique. Grâce au financement Innogap, le groupe du Prof. Gerrit Borchard explorera une nouvelle méthodologie pour l’administration simultanée et possiblement synergique de ces composés. Photo, de gauche à droite: Prof. Gerrit Borchard, Katrin Fuchs et Dr Olivier Jordan.

Un nouveau bracelet pour optimiser l'observance de l’hygiène des mains en milieu hospitalier (Yves Martin, Ingénieur HUG)
Les maladies nosocomiales sont responsables de 2'000 décès par an en Suisse. Cela génère CHF 350 millions de dépenses supplémentaires. Une hygiène des mains appropriée permet de réduire ces infections d’au moins 50%. L’ingénieur Yves Martin, co-inventeur de cette technologie, travaille étroitement avec le Prof. Didier Pittet aux HUG afin de développer des systèmes permettant d’améliorer l’observance de l’hygiène des mains en milieu hospitalier. Son invention consiste en un bracelet permettant de détecter en temps réel le lavage des mains et d’un dispositif équipant les bouteilles de solution hydro-alcoolique permettant de mesurer la quantité de solution extraite des bouteilles. Le financement INNOGAP permettra de corriger certains défauts identifiés lors d’un récent test pilote sur pré-prototypes. Les HES-SO de Genève et du Valais qui sont co-propriétaires de la technologie seront mandatés pour réaliser ces améliorations. De gauche à droite: Simon Fourquier (Hepia), René Beuchat (Hepia), Yves Martin (HUG) et Gilbert Maître (HES-SO Valais).
FUNDED PROJECTS IN 2013
FALL 2013

Une nouvelle méthode visant une détection précoce des pathologies liées à des accidents cérébraux ( Professeur Jean-Charles Sanchez )
Avec son équipe de la Faculté de médecine de l’UNIGE, le Professeur Jean-Charles Sanchez travaille depuis plusieurs années à l’identification de biomarqueurs liés à des pathologies cérébrales. Le Professeur Sanchez et son équipe vise aujourd’hui à définir des tests efficaces avec un panel de biomarqueurs spécifiques qui permettrait de diagnostiquer de manière précoce, chez des patients présentant des signes modérés de traumatismes cérébraux, la gravité de leur état ainsi que le traitement adéquat. Photo: Prof. Jean-Charles Sanchez et Linnéa Lagerstedt.

De nouveaux matériaux pour les semi-conducteurs de demain (Dr. Alberto Ubaldini/ Dr. Enrico Giannini)
L’industrie des semi-conducteurs s’intéresse aux nouveaux matériaux permettant de remplacer à terme le silicium. Le but est à la fois de permettre une miniaturisation encore plus grande des transistors, mais également d’anticiper l’épuisement du silicium. Alberto Ubaldini, au département de physique de la matière condensée a inventé un procédé permettant de produire des cristaux à base de dichalcogénures de métaux de transition. La structure atomique de ces matériaux est MX2 où (M = Ti, Zr, Hf, V, Nb, Ta, Mo, W et X = S, Se,Te) et la synthèse est très délicate sur le plan technique. Le groupe d’Alberto Ubaldini (UNIGE/ DPMC) bénéficie d’une bourse INNOGAP pour acquérir un four permettant de produire ces cristaux de manière reproductible et à plus grande échelle. Le Dr Enrico Giannini (photo devant le nouveau four) est en charge de ces développements.http://www.q-mat.ch/.
SPRING 2013

Code-barres génétiques pour le diagnostic écologique des écosystèmes aquatiques (Professeur Jan Pawlowski)
Le groupe du Prof. Jan Pawlowski travaille sur l’évolution et l’écologie moléculaires des oligochètes en utilisant des données génomiques. Les oligochètes sont des eucaryotes unicellulaires, souvent utilisés comme bio-indicateur pour l’environnement afin d’évaluer la qualité des sédiments et de l’eau. Actuellement, l’identification de ces espèces s’effectue morphologiquement sous microscope. Le financement INNOGAP a pour objectif de remplacer ce travail fastidieux par un test génétique automatisé pour évaluer plus rapidement la qualité écologique des écosystèmes aquatiques. Ce test plus fiable et moins coûteux sera proposé aux services de protection et de gestion de ressources aquatiques en Suisse et à l’étranger
BARD1 targeting cancer therapeutic (Dr Irmgard Irminger-Finger)
Le groupe de Dr. Irmgard Irminger-Finger aux HUG travaille depuis de nombreuses années sur l’implication de la protéine BARD1 dans les mécanismes moléculaires liés au cancer. En effet, la protéine normale protège les cellules du cancer, alors que certains variants de cette protéine semblent au contraire causer des cancers. Le groupe du Dr. Irmgard Irminger-Finger a conçu un agent qui pourrait bloquer l’action de ces variants oncogènes de la protéine BARD1 et de ce fait agir comme agent anti-cancéreux. Le financement INNOGAP vise à mettre au point la production du composé et étudier sa capacité à inhiber la proflifération des cellules tumorales en culture.
FUNDED PROJECTS IN 2012
FALL 2012

The mitochondrial pyruvate carrier (Professeur Jean-Claude Martinou)
Le Prof. Jean-Claude Martinou et le Dr. Sébastien Herzig du département de biologie cellulaire ont identifié une nouvelle cible moléculaire qui se situe au cœur des voies du métabolisme cellulaire et pourrait être impliquée dans diverses pathologies, comme le cancer du sein ou les lésions liées à la reperfusion après ischémie. Le financement Innogap de CHF 30'000 sera dédié à la recherche de petites molécules inhibant cette cible et à l’évaluation de leur potentiel thérapeutique dans divers modèles cellulaires.
Sur la photo: (de gauche à droite debout) Christopher Rodley, Alexis Jourdain, Audrey Bellier, Professeur Jean-Claude Martinou, Sylvie Montessuit, Erik Boehm, Monique Fornallaz. (Accroupies) Vincent Compan, Gabrielle Penna, Thomas Landes, Sébastien Hertig.

Conception d’un nouvel implant vestibulaire (Professeur Marco Pelizzone)
Le Prof. Marco Pelizzone et ses collaborateurs du service d’ORL et chirurgie cervico-faciale des HUG ont conçu un nouvel implant vestibulaire basé sur la technologie existante de l’implant cochléaire pour restituer le reflexe vestibulo-oculaire. Ceci permettrait à certains patients de retrouver une stabilisation satisfaisante du regard dans les situations dynamiques. Le subside INNOGAP de CHF 30'000 permettra de produire un prototype portable pour effectuer des essais cliniques.
Sur la photo: le Professeur Jean-Philippe Guyot (deuxième depuis la gauche), le Professeur Marco Pelizzone (troisième depuis la gauche), Samuel Cavuscens, Maurizio Ranieri, Nils Guinand, Angelica Perez.
SPRING 2012
Un nouveau senseur de ions électrochimique (Professeur Eric Bakker)
Le Prof. Eric Bakker (avec Alexey Shvarev à sa droite) a inventé un senseur de ions électrochimique très avantageux. Il permet de mesurer précisément la concentration de ions en solution (Calcium, Potassium, Sodium, etc) par la méthode coulometrique par contrôle du potentiel. La méthode mesure les concentrations ioniques en continu par extraction à travers une membrane sélective et ne nécessite que de très petits volumes de solution. Les perspectives d’application de la méthode sont multiples et touchent non seulement les instruments de laboratoire mais aussi des instruments portables utilisables sur le terrain. Le subside INNOGAP de CHF 29'575 permettra de produire un prototype du modèle « portable ».

Un nouveau détecteur d'aérosols à fort potentiel (Dr. Denis Kiselev)
Denis Kiselev, chercheur au département de physique appliquée a inventé un efficace détecteur d’aérosols. Il fonctionne avec des lasers qui stimulent la diffusion lumineuse et la fluorescence des particules d’aérosols qui traversent le détecteur. La signature fluorescente est résolue spectralement par un photomultiplicateur à 32 canaux. Ce détecteur permettra de caractériser la taille, la forme et la composition des particules uniques d'aérosols. Les applications comprennent la détection en temps réel de pollens (allergies), la détection de pathogènes (santé, sanitaire) ou encore la détection substances chimiques ou biologiques (sécurité, terrorisme) de taille macroscopique. Le subside INNOGAP de CHF 25'000 permettra de fabriquer le premier prototype pour les mesures hors laboratoire.
Système d'aide à la propulsion pour fauteuil roulant (Dr. Stéphane Armand)
Monsieur Armand et Yoshimasa Sagawa, à sa droite, ont inventé un nouveau système de propulsion pour chaise roulante. Si la méthode fonctionne, les handicapés pourront se déplacer plus confortablement et à moindre effort. Le subside INNOGAP de CHF 15'000 permettra de réaliser une preuve du concept.

Nouveau dispositif médical pour traiter les infections de la cornée (Professeur Farhad Hafezi et Dr. Olivier Richoz
Prof. Farhad Hafezi (à droite) du département des neurosciences cliniques et chef de service d’ophtalmologie des HUG et le Dr. Olivier Richoz ont inventé un nouveau dispositif médical pour traiter des maladies et les infections de la cornée. Le dispositif a pour fonction de photoactiver la riboflavin et générer des radicaux libres. Les radicaux libres permettent d’augmenter la rigidité de la cornée en induisant le « crosslinking » du collagène et d’éliminer les agents infectieux. Le subside INNOGAP de CHF 30'000 permettra de fabriquer un prototype avec un marquage CE pour initier des tests précliniques.
FUNDED PROJECTS IN 2011
Complexe moléculaire permettant la conversion de lumière infra-rouge en lumière visible (Upconversion) (Professeur Claude Piguet et Professeur Andreas Hauser)
L'invention des Professeurs Piguet et Hauser décrit un nouveau complexe moléculaire métallique qui est capable de convertir la lumière infra-rouge en lumière visible. Cette propriété unique au monde au niveau moléculaire a un fort potentiel pour la bio-imagerie ou encore pour les cellules photo-voltaïques.

Appareil de synthèse miniaturisé pour la préparation de radiopharmaceutiques (Professeur Yann Seimbille)
Le groupe du Prof. Yann Seimbille a pour projet de fabriquer un appareillage miniaturisé en utilisant des microchips stériles et jetables pour marquer des molécules biologiques avec divers radioisotopes. Cet appareil aura pour avantage de réduire le temps des synthèses des radiopharmaceutiques et les produire à des quantités plus adaptées pour des utilisations précliniques et cliniques.

Nouvelle thérapie contre le cancer (Professeur Ruiz i Altaba)
Les chercheurs ont identifié une nouvelle cible cellulaire pour laquelle l'Université de Genève a déposé une demande de brevet. Cette cible pourrait permettre le développement de nouveaux ou meilleurs traitements contre le cancer. Suite à d'encourageantes évaluations in-vitro, les chercheurs évalueront différentes formulations de traitement en utilisant un modèle in-vivo de tumeur cérébrale humaine sur des souris. Les données in-vivo seront primordiales pour susciter l'intérêt de l'industrie pharmaceutique dans le but de développer un produit thérapeutique.

Un nouveau vecteur pour l’immunothérapie contre le cancer (Dr. Madiha Derouazi, Dr. Paul Walker, Prof. Pierre-Yves Dietrich)
Pour qu’une immunothérapie contre le cancer soit efficace, il faut que l’antigène tumoral soit présenté aux cellules T cytotoxiques et aux cellules T auxiliaires : les cellules T cytotoxiques vont tuer les cellules cancéreuses, et les cellules T auxiliaires vont les aider à y parvenir.
Dr. Madiha Derouazi, Dr. Paul Walker et Prof. Pierre-Yves Dietrich ont développé un nouveau vecteur de vaccination – un fragment de la protéine ZEBRA du virus Epstein-Barr - qui permet une présentation simultanée de plusieurs antigènes aux cellules T cytotoxiques et auxiliaires, ce qui le rendrait particulièrement utile pour l’immunothérapie contre le cancer.
L’équipe va utiliser le financement INNOGAP pour avancer vers une preuve de concept chez l’animal, à savoir démontrer que des antigènes introduits grâce au vecteur ZEBRA peuvent induire une réponse immune multi-épitopique et réduire la taille de tumeurs dans la souris. Une fois cette étape franchie, l’équipe envisage de créer une entreprise qui se baserait sur cette technologie pour développer des vaccins contre le cancer.

Un nouvel agent antibactérien (Dr. Sara Deakin, Prof. Richard James)
Pseudomonas aeruginosa et d’autres bactéries à Gram négatif causent des infections nosocomiales, en particulier chez des patients qui ont des problèmes respiratoires (par exemple des patients ventilés) and chez les grands brûlés. Pour qu’une infection à Pseudomonas chronique devienne virulente, c'est-à-dire dangereuse pour le patient, des bactéries individuelles doivent communiquer entre elles par un mécanisme connu sous le nom de « quorum sensing ».
Dr. Sara Deakin et Prof. Richard James ont conçu un agent qui pourrait inhiber le quorum sensing et empêcher les bactéries de devenir virulentes.
Le financement INNOGAP sera utile pour mettre au point la production du composé et étudier sa capacité à inhiber le quorum sensing dans un modèle d’infection bactérienne.

Nouvelle approche dans l'administration de médicaments pour le traitement des cancers de la région tête/cou (Dr. Tais Gratieri, Dr. Yogeshvar Kalia)
Ce projet propose une nouvelle approche pour la délivrance ciblée de médicaments anticancéreux aux tumeurs localisées dans la région tête/cou. Actuellement, lors de traitement de telles tumeurs, les médicaments sont généralement administrés de manière systémique.
L'administration locale développée par le groupe du Dr. Kalia offre l'opportunité de délivrer de hautes concentrations de médicaments directement au site de la tumeur à des doses moindres permettant ainsi des effets thérapeutiques améliorés et des effets indésirables réduits sur d'autres tissus.
FUNDED PROJECTS IN 2010
Nouvelle méthode pour la réalisation du processus de PEGylation (Prof. Gerrit Borchard)
La PEGylation est une méthode qui modifie des molécules, notamment des composés thérapeutiques, par l'attachement covalent de chaînes de poly (éthylène-glycol), dites PEG. Ce processus permet d’améliorer le profil pharmacocinétique, toxicologique et immunologique de ces composés. Cette méthode est aujourd’hui largement reconnue et utilisée par les entreprises pharmaceutiques.
Au travers du subside UNIGAP d’un montant de 25’500CHF qui leur a été octroyé, Le Professeur Gerrit Borchard et son équipe souhaitent développer de nouvelles méthodes afin de réaliser le processus de PEGylation de manière non-covalente, ce qui signifie que des molécules peuvent être PEGylées sans modification chimique et sans changements concomitants des caractéristiques chimiques et thérapeutiques de la molécule.
Une telle méthode pourrait améliorer la performance ainsi que les coûts de production de ces composés thérapeutiques.
Développement d’un interface cerveau-machine low-cost (Dr Sara Gonzalez Andino et Dr Rolando Grave de Peralta)
Les deux chercheurs de la faculté de médecine et leur équipe ont développé un interface cerveau-machine qui permet au cerveau de communiquer directement avec le monde extérieur sans passer par les muscles ou les nerfs. Cette technologie serait particulièrement utile pour les malades à motricité limitée. Elle permettrait notamment de piloter une chaise roulante, d’activer des télécommandes ou de téléphoner.
Vu les performances de la technologie en termes de vitesse, de fiabilité et de temps d’apprentissage, elle offre aussi de belle perspectives dans d’autres domaines comme la réalité augmentée. Le subside UNIGAP de CHF 7'000 complétera le budget disponible et permettra à HES-Arc et aux HUG de développer de manière collaborative un casque EEG « low cost » qui pourra s’appliquer à plusieurs domaines.
Le principe de fonctionnement est que lorsque les yeux se focalisent sur une surface d’écran (carré de 5cm par 5cm par exemple) oscillant à une certaine fréquence, c’est une partie spécifique du cerveau qui s’active à la même fréquence. On mesure cette activation avec des électrodes placées sur la tête (casque EEG) ; des commandes peuvent en être déduites.
Nouveaux microbicides contre le virus du Sida (Prof. Jeremy Luban)
Chaque année, des millions de personnes sont infectées par le virus du Sida dont la grande majorité vit dans les pays en voie de développement. Pour de nombreuses raisons, peu de femmes sont protégés par l’utilisation des préservatifs dans ces pays.
Des études cliniques récentes ont démontré que les microbicides applicables sur les muqueuses génitales sont un moyen efficace pour protéger les femmes contre le virus du Sida.
Le groupe du Prof. Jeremy Luban a découvert que des substances biologiques fabriquées à un faible coût inhibent fortement les infections des lymplocytes par le Sida. Ces substances pourraient être utilisées seules ou en combinaison avec d’autres molécules anti-Sida pour constituer un nouveau microbicide.
Les expériences proposées avec le subside UNIGAP de CHF 30'000 ont pour objectifs de confirmer l’efficacité et la spécificité de l’inhibition des substances biologiques in vitro en utilisant des souches primaires de virus (non de laboratoire) et les cellules cibles humaines du virus (lymphocytes, macrophages et dendrites).
Développement thérapeutique contre le syndrome métabolique (Prof. Françoise Jeanrenaud-Rohner)
Le syndrome métabolique, dont près d’un quart de la population adulte souffre, est défini par une coïncidence de plusieurs facteurs de risques tels que glucose élevé, obésité abdominale, cholestérol élevé et pression sanguine élevée. Pour beaucoup de patients, le syndrome métabolique progresse vers des maladies graves comme le diabète de type 2 ou des maladies cardiovasculaires.
Actuellement, chaque facteur de risque doit être traité individuellement, ce qui force les patients à prendre beaucoup de pilules. A cause de la charge qui en résulte pour l’organisme, et du fait que certains de ces traitements peuvent interférer entre eux, le plus souvent seulement une partie des facteurs de risque peut être traitée pour un patient donné.
Le groupe du Prof. Françoise Jeanrenaud a découvert qu’une hormone endogène, l’ocytocine, a un effet bénéfique sur tous les aspects liés au syndrome métabolique. Un médicament à base d’ocytocine pourrait ainsi traiter de manière idéale le syndrome métabolique et éviter qu’il ne progresse vers des maladies grave. Toutefois, l’ocytocine a une durée de vie très courte dans la circulation et n’existe qu’en formulation injectable, ce qui rend son administration chronique peu attractive. Un subside UNIGAP a été octroyé au groupe du Prof. Jeanrenaud pour tester dans un modèle animal du syndrome métabolique l’activité d’analogues plus stables de l’ocytocine qui pourraient être administrés sous forme de spray ou par voie orale.
Développement d'inhibiteurs des kinases ALK pour des traitements anti-cancéreux (Prof. Leonardo Scapozza)
Les membres de la famille des kinases ALK sont des cibles reconnues pour le développement de médicaments contre la majorité des lymphomes anaplasiques à grandes cellules et certains autres cancers tels que le cancer du poumon non à petites cellules, le neuroblastome ou les tumeurs inflammatoires myofibroblastiques. L’équipe du Prof. Scapozza en collaboration avec ses collègues de l’Université de Lyon et de Milan, a conçu et développé quatre nouvelles classes de composés chimiques qui sont capables d’entraver avec une grande efficacité l’activité des ALK dans des modèles cellulaires. Sur la base de ces études, une douzaine de molécules candidates ont été identifiées qui pourraient être prometteuses pour le développement d’un médicament anti-cancéreux.
Pour l’instant, les molécules candidates identifiées sont encore trop nombreuses pour être toutes testées en clinique. Le subside UNIGAP permettra à l’équipe du Prof. Scapozza de raffiner et restreindre la sélection grâce à des techniques de pointe de modélisation moléculaire. Ainsi, seules les molécules les plus prometteuses seront synthétisées pour être testées d’abord sur l’animal, puis finalement sur l’homme.
FUNDED PROJECTS IN 2009
Administration ciblée de médicaments (Dr. Andreas Zumbuehl)
Ce projet repose sur le concept de l'administration de médicaments dans le système circulatoire d'un patient au moyen d'une structure relâchant un composant actif sur un site thérapeutique précis. Un tel processus interviendrait via les caractéristiques physiologiques, biochimiques ou environnementales d'un organe ou d'un tissu visé par la molécule active. Ce concept permettrait d'offrir des thérapies ciblées par administration systémique tout en réduisant, voire éliminant les effets secondaires indésirables sur d'autres organes ou des tissus. Ce projet offrirait une valeur commerciale notable étant donné son potentiel pour l'administration de nouvelles molécules biologiques actives aux sites thérapeutiques souhaités et pour l'amélioration du cycle de vie de produits thérapeutiques existants. Le travail financé par UniGAP se concentrera sur la mise au point, la caractérisation et la validation de ces structures ayant des qualités spécifiques liées au transport et à l'administration de molécules biologiques actives sur des sites thérapeutiques précis.
Microbioréacteur capillaire (Prof. Jean-Luc Veuthey)
Le développement de produits thérapeutiques nécessite d'effectuer des tests coûteux de toxicité afin de définir la stabilité ou la vitesse de dégradation d'un médicament dans le corps humain. Ces études permettent de définir le dosage approprié (efficace sans être toxique) d'un médicament que l'on peut administrer aux patients.
Le groupe du Prof. Jean-Luc Veuthey à la Faculté des Sciences a développé un microbioréacteur capillaire pour automatiser les réactions de métabolisme des médicaments dans des petits tubes capillaires à très faible volume. Cette technique innovante devrait réduire les coûts des études de métabolisme de médicaments par un facteur cent.
Un premier prototype de ce microbioréacteur capillaire sera réalisé grâce au financement UNIGAP en collaboration avec la HES-SO.


