« Back to all publications
Download this list in a RIS file or a BIB file or a PDF file
|
 |
|||||||
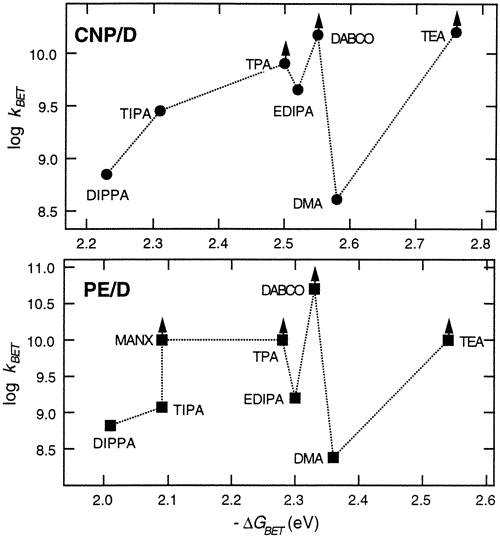
The dynamics of charge recombination within geminate ion pairs formed by electron transfer (ET) quenching of excited aromatic hydrocarbons by aliphatic and aromatic amines was investigated using picosecond transient grating spectroscopy. With aliphatic donors, the rate constant of back ET, kBET, shows a substantial decrease with increasing steric encumbrance around the N atom. No correlation between kBET and the exergonicity of the process was observed. This effect is ascribed to a decrease of the electronic coupling matrix element, V, which is affected by both the distance between the N atom of the donor and the aromatic plane of the acceptor and by the delocalization of the hole upon increasing the bulkiness of the alkyl substituents. With aromatic amines, kBETis substantially slower than with the unhindered amines. This is also explained in terms of a smaller value of V because of charge delocalization. | ||||||||
|
||||||||
The deuterium isotope effect on the fluorescence lifetime of contact ions pairs (CIP) composed of 9,10-dicyanoanthracene and weak aromatic electron donors in acetonitrile has been investigated. For all pairs studied, an increase of the emission lifetime with increasing deuterium substitution on both the electron acceptor and the electron donor was observed. The free ion yield shows the same variation upon deuteration. It is concluded that the efficiency of free ions formation is essentially determined by the competition, within the CIP, between charge recombination and dissociation into free ions and that loose ion pairs do not play a significant role. | ||||||||
|
|
Download this list in a RIS file or a BIB file or a PDF file
Contact:
Eric Vauthey
Physical Chemistry Department - Sciences II - University of Geneva
30, Quai Ernest Ansermet - CH-1211 Geneva 4 (Switzerland)
© All rights reserved by Eric Vauthey and the University of Geneva
Design and code by Guillaume Duvanel




