|
Photophysics and Photochemistry of Transition Metal Compounds |
| Home Research Members Collaborations Publications |
1) TTF-dppz light induced intraligand charge transfer
The photophysical and photochemical properties of TTF have been longly studied during the past four decades.
TTF is qualified as a luminescence quecher and a good electron donor. The light induced electron transfer is one
of the most important photophysical processes since the photosynthesis and the cellular respiration relay on it.
In our group, we extensively study photophysical and photochemical properties of several ligands linked to the TTF moiety, containing or not a metal ion.
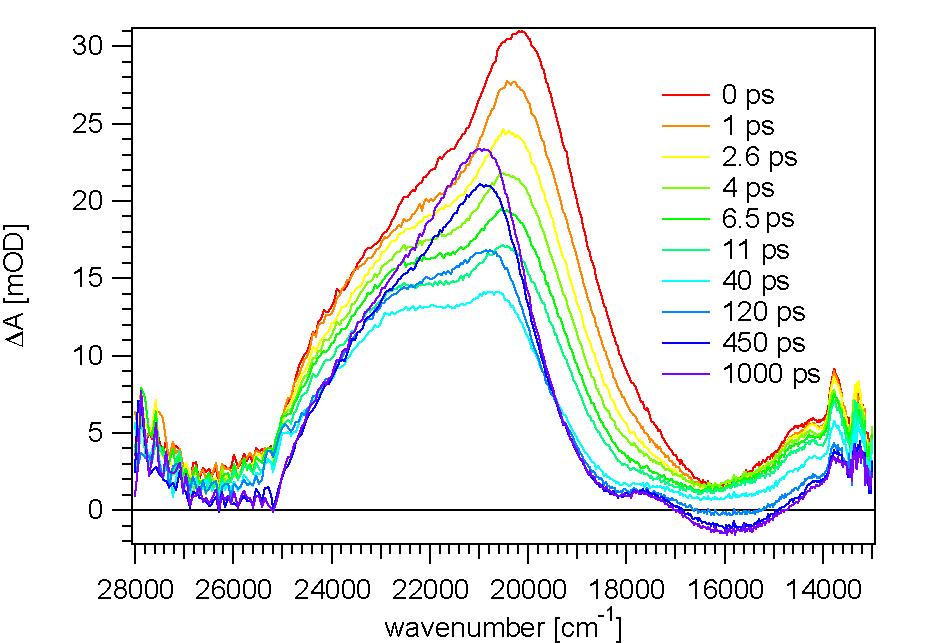
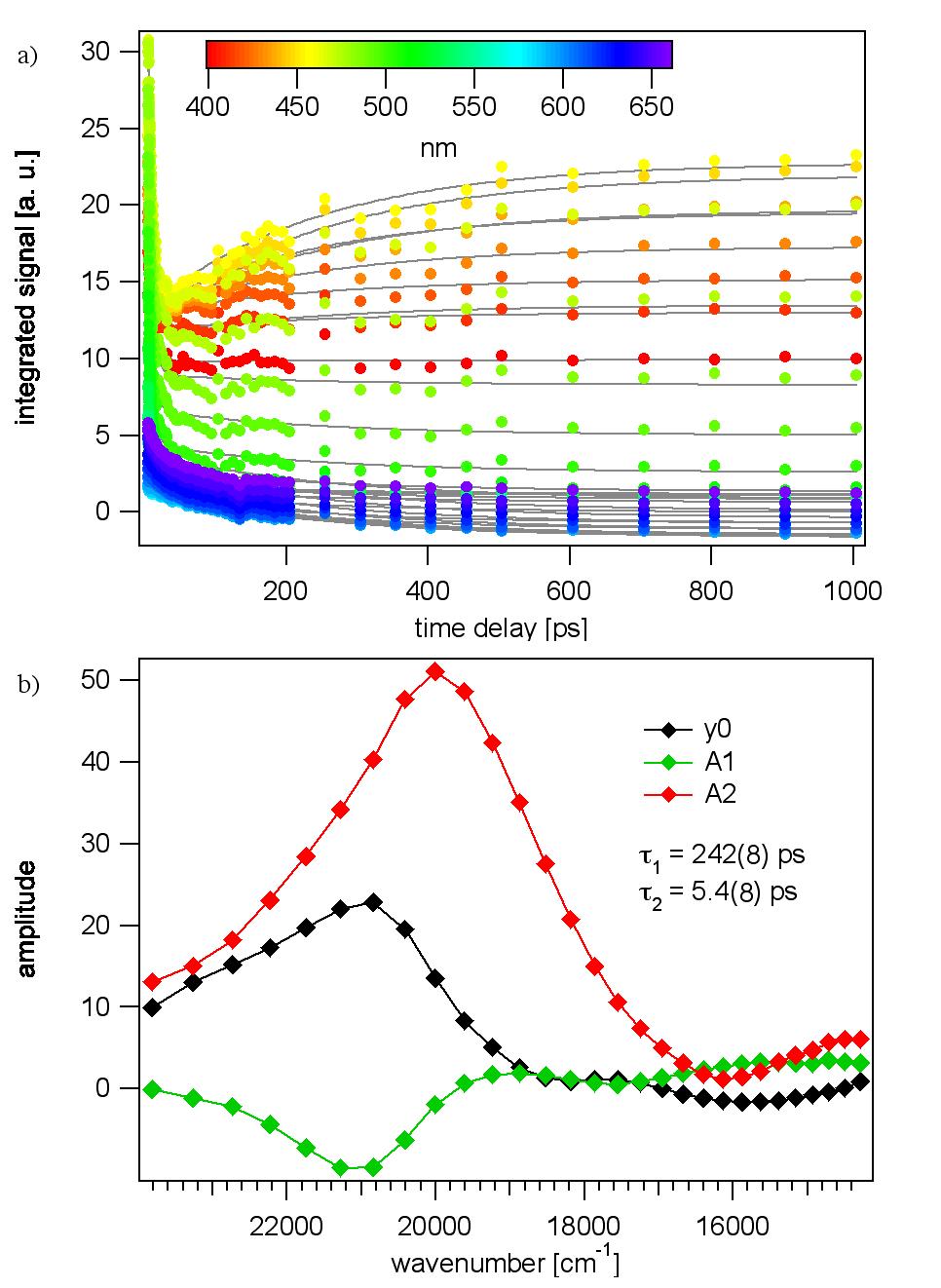
It appears that the TTF-dppz ligand undergo an intraligand charge transfer, the electron going from TTF to dppz. The creation of long-lived charge-separated states in donor-acceptor assemblies has been
the goal of many studies aimed at mimicking the primary processes in photosynthesis. Here we
present such assemblies based on tetrathiafulvalene (TTF) as electron donor and a dipyridophenazine
(dppz) unit as electron acceptor in the form of a fused ligand (TTF-dppz) coordinated to
ruthenium(II) via the dipyrido coordination site and with 2,2'-bipyridine (bpy) as auxiliary ligand,
namely [Ru(bpy)(3-x)(TTF-dppz)(x)](2+) (x = 1-3). For x = 2, irradiation into the metal to dppz
charge transfer transition results in electron transfer from TTF to ruthenium, thus creating a chargeseparated
state best described by [(TTF+-dppz)Ru(dppz(-)-TTF)(bpy)]2+ with a lifetime of 2.5 μs
in dichloromethane.

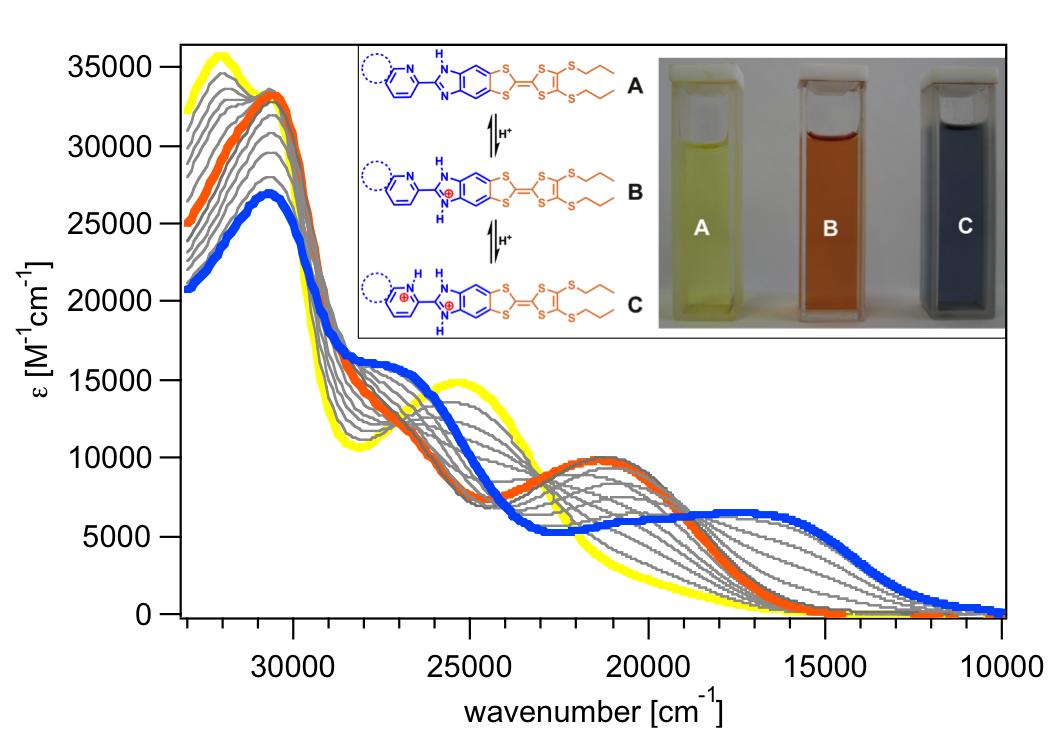
In order to study the electronic interactions in donor-acceptor ensembles as a function of
pH, an efficient synthetic route to three imidazole-annulated tetrathiafulvalene (TTF) derivatives 1-3
is reported. Their electronic absorption spectra, in view of photoinduced intramolecular charge
transfer, and their electrochemical behavior were investigated, and pK(a) values for the two
protonation processes on the acceptor unit were determined in organic solvents by photometric
titration. The influence of the TTF moiety on these values is discussed.
The synthesis and the photophysical properties of the complex [Ru(TTFdppz)
2(Aqphen)]2+ (TTF = tetrathiafulvalene, dppz = dipyrido-[3,2-a:2',3'-c]phenazine, Aqphen =
anthraquinone fused to phenanthroline via a phenazine bridge) are described. In this molecular triad
excitation into the metal-ligand charge transfer bands results in the creation of a long-lived charge
separated state with TTF acting as electron donor and anthraquinone as terminal acceptor. The
lifetime of the charge-separated state is 400 ns in dichloromethane at room temperature. A
mechanism for the charge separation involving an intermediate charge-separated state is proposed
based on transient absorption spectroscopy