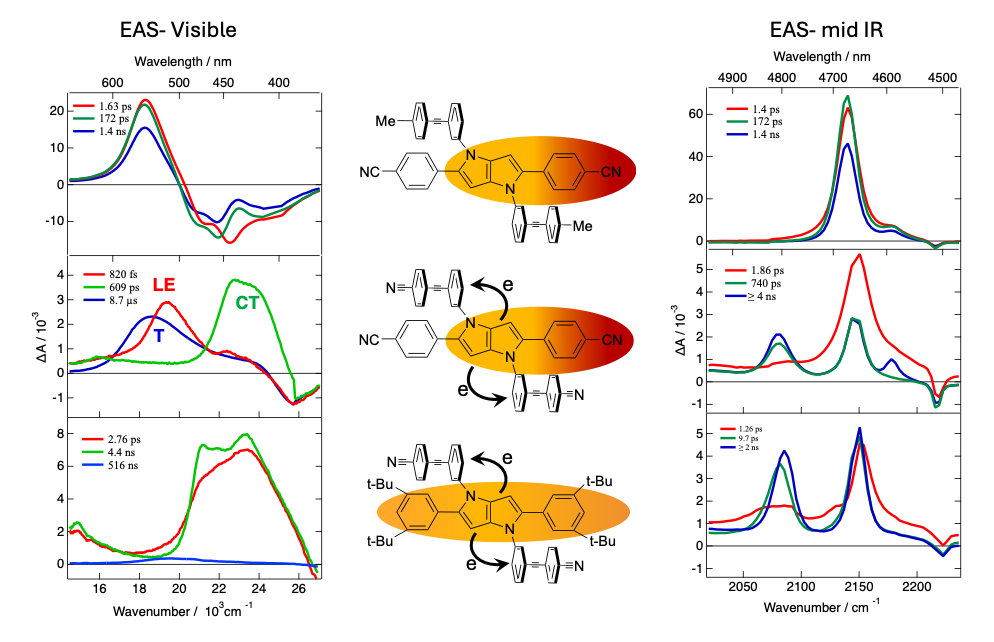
We are investigating excited-state charge transfer and symmetry breaking in a series of quadrupolar pyrrolopyrrole-based molecules, synthesised in the Gryko group in Warsaw, with donor (D) and acceptor (A) groups of varying strength using a combination of transient electronic and vibrational absorption spectroscopies.
The left and right figures illustrate the evolution-associated difference absorption spectra (EAS) obtained from a global analysis of the transient absorption data. With weak A and D (top panels), the -C≡C- stretching band does not appear in the transient vibrational spectrum, implying that electronic excitation remains on the pyrrolopyrrole core. In polar solvents, an asymmetric distribution of the excitation in the core is observed. By contrast, ultrafast intramolecular charge transfer (ICT) followed by intersystem crossing (ISC) is observed with either a stronger A or a weaker D (mid and bottom). ICT breaks the symmetry further as an electron is transferred on a single branch. The dynamics of this process and of the ensuing recombination depend significantly on the driving force.
| FOR MORE INFORMATION | ||
Contact:
Eric Vauthey
Physical Chemistry Department - Sciences II - University of Geneva
30, Quai Ernest Ansermet - CH-1211 Geneva 4 (Switzerland)
© All rights reserved by Eric Vauthey and the University of Geneva
Design and code by Guillaume Duvanel



