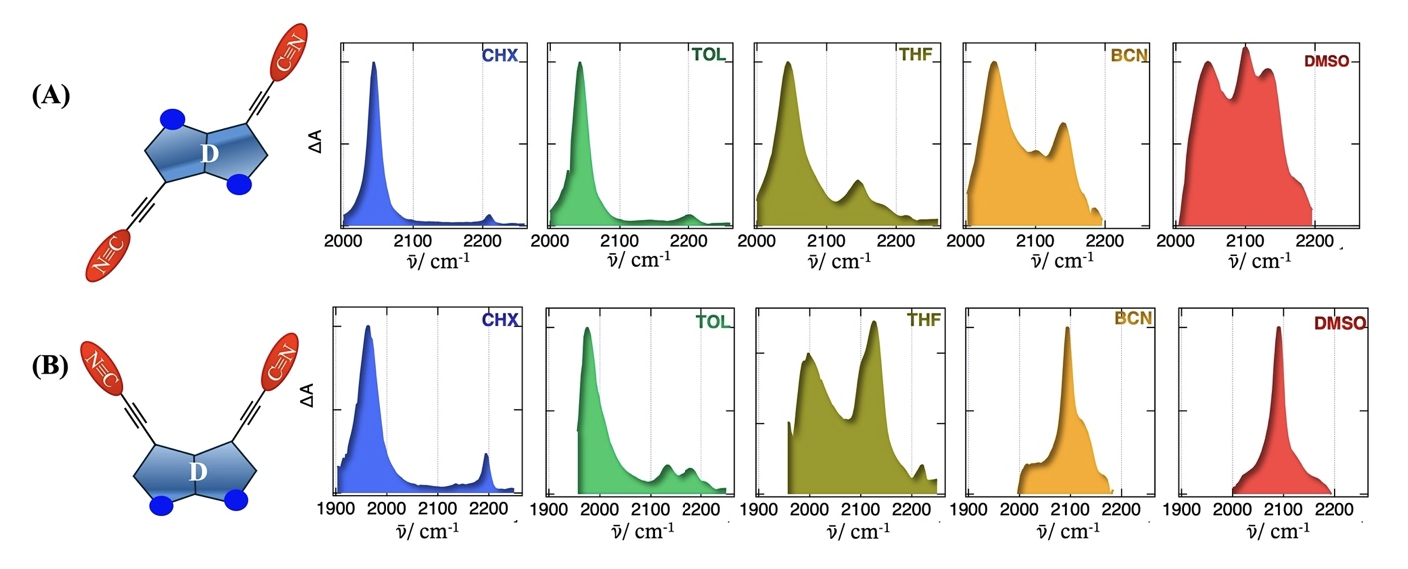
We are investigating excited state-symmetry breaking (ES-SB) in A-π-D-π-A triads with a strongly electron-donating indolo[3,2-b] indole or indolo[2,3-b]indole core synthesized in the group of Dr. Jubi John in India. The above figure shows the evolution associated spectra corresponding to the relaxed S1 state obtained from global analysis of the time-resolved IR data of dyes (A) and (B) with increasing solvent polarity. In (A), the presence of a single -C≡C- band around 2050 cm⁻¹, and a single -C≡N band around 2210 cm⁻¹ in the nonpolar cyclohexane points to an electronic excitation delocalised over both branches. The occurrence of band splitting upon increasing solvent polarity is attributed to the symmetry-broken state. However, full localisation of the exciton on a single branch does not occur even in the highly polar DMSO. Interestingly, in the non-centrosymmetric dye (B), the spectral evolution is completely different compared to (A). We are performing further studies, including quantum chemical calculations, to rationalise these observations.
| FOR MORE INFORMATION | ||
Contact:
Eric Vauthey
Physical Chemistry Department - Sciences II - University of Geneva
30, Quai Ernest Ansermet - CH-1211 Geneva 4 (Switzerland)
© All rights reserved by Eric Vauthey and the University of Geneva
Design and code by Guillaume Duvanel



