Open-shell radical ions are the product of the electron transfer reaction between two neutral molecules. Although excited open-shell organic ions have been invoked to account for phenomena as diverse as the absence of inverted region in highly exergonic electron transfer quenching reaction or the emissive diffuse interstellar bands, their properties are almost unexplored. A major reason for the lack of information on these species is certainly the absence of fluorescence from most radical ions in the condensed phase. Moreover, many emissions ascribed to radical ions in solution are in fact due to secondary closed shell products.
In order to get more information on these species, we are investigating their excited state dynamics using principally the transient grating technique. The radical ions are formed in different ways:
- chemically: by oxidation in concentrated sulfuric acid;
- radiolytically: by g irradiation in low temperature matrices;
- photochemically: by photoinduced electron transfer reaction in acetonitrile or by ionisation in a boric acid glass.
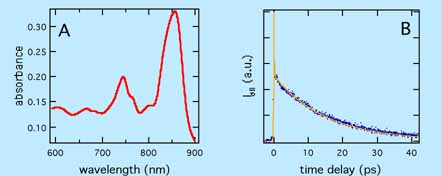
A) Absorption spectrum of tetracene in sulfuric acid; B) Dynamics of ground state recovery of tetracene cation measured by transient grating at 840 nm
Until now we have only investigated a few radicals (cations: perylene, tetracene and thianthrene; anions: perylene, anthraquinone and tetracenequinone). The excited lifetime of these radicals was found to be very short in liquids (< 30 ps, see above figure). In sulfuric acid, this is mainly due to the occurrence intermolecular quenching processes, whose mechanism is still unclear. In acetonitrile, the excited state lifetime is even shorter (< 15 ps) for reasons, which are also not fully understood. Apart from these lifetimes, we have observed for the first time the absorption band of a radical cation (tetracene cation) in the excited state and the D2-D0 fluorescence of a radical anion (exception of Kasha's rule).
Contact:
Eric Vauthey
Physical Chemistry Department - Sciences II - University of Geneva
30, Quai Ernest Ansermet - CH-1211 Geneva 4 (Switzerland)
© All rights reserved by Eric Vauthey and the University of Geneva
Design and code by Guillaume Duvanel

