- Mora, F.; Tran, D.-H.; Oudry, N.; Hopfgartner, G.; Jeannerat, D.; Sakai, N.; Matile, S. “Interface Engineering of Synthetic Pores: Toward Hypersensitive Biosensors” Chem. Eur. J. 2008, 14, 1947-1953
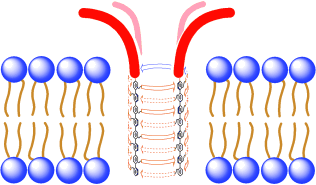
Hydrophilic anchoring is introduced as a promising strategy to constructively control the various interactions of synthetic pore sensors with the surrounding biphasic environment. Artificial rigid-rod β barrels are selected as classical synthetic multifunctional pores and random-coil tetralysines are attached as hydrophilic anchors. The synthesis of this advanced pore is accomplished in 32 steps from commercially available starting materials. With regard to pore activity as such, the key impact of hydrophilic anchoring is a change from a Hill coefficientnn=4. This change confirms successful suppression of the competing self-assembly with precipitation from the aqueous phase as the origin of the accomplished increase in pore activity. The hydrophilic anchors do not interfere with the blockage of the synthetic pore sensors by anionic analytes. In the case of stoichiometric binding of blockers (KD=EC50 of the pore; EC50=concentration needed to observe 50 % pore activity), however, the increase in pore activity achieved by hydrophilic anchoring results in improved pore blockage under high dilution conditions. Controls confirm that this increase does not occur with analytes that do not exhibit stoichiometric binding (KD>EC50). These results not only reveal stoichiometric binding as the expected origin of the sensitivity limit of synthetic pore sensors, they also provide promising solutions for this problem. The combination of hydrophilic anchoring with targeted pore formation emerges as a particularly promising strategy to further reduce effective pore concentrations. The scope and limitations of this approach are exemplified with pertinent analyte pairs that are essential for the sensing of sucrose, lactose, acetate, and glutamate with synthetic pores in samples from the supermarket.
