- Verolet, Q.; Dal Molin, M.; Colom, A.; Roux, A.; Guénée, L.; Sakai, N.; Matile, S. “Twisted Push-Pull Probes with Turn-On Sulfide Donors” Helv. Chim. Acta 2017, 100, e1600328
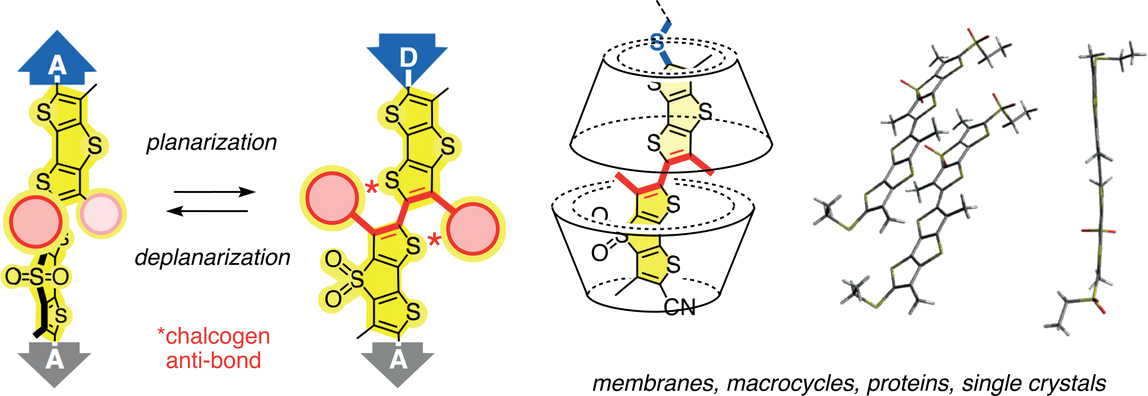
Planarizable and polarizable dithieno[3,2-b;2’,3′-d]thiophene (DTT) dimers have been introduced recently as fluorescent probes that report on membrane fluidity with red shifts in excitation, i.e. planarization in the ground state. In this study, we elaborate on the hypothesis that twisted push-pull probes could perform best in the presence of one unorthodox substituent that acts as a weak acceptor with electron-rich and as a strong donor with electron-poor aromatics. According to Hammett constants, we thought that sulfides could provide access to such a conceptually innovative donor-acceptor switch. To elaborate on this hypothesis, we here describe the design, synthesis and evaluation of a comprehensive series of twisted push-pull probes with turn-on sulfide donors. Their planarization is explored in lipid bilayer membranes of different thickness and fluidity from liquid-disordered to liquid-ordered and solid-ordered phases. Results from membranes are compared to the planarization of turn-on mechanophores in crystals, proteins and cyclodextrin macrocycles of varied diameter.
open archive unige:91890 • pdf ![]()
