Repairing synapses to combat multiple sclerosis
A team from UNIGE, the University of Munich and the Technical Institute of Munich has discovered that the destruction of synapses in the brain's grey matter reduces the activity of neurons in the cerebral cortex and is a major factor in the progression of multiple sclerosis.
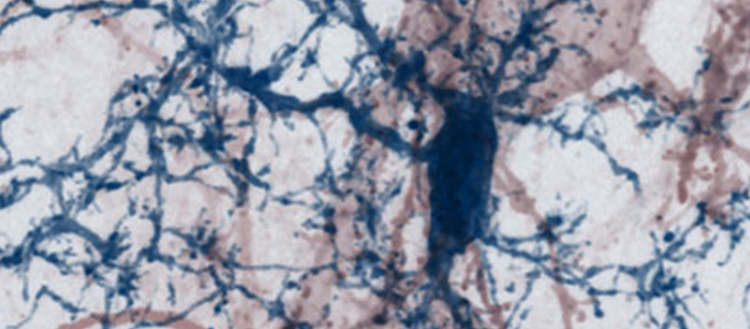
© Microscopy image from mouse brain showing a nerve cell with its dendritic processes studded by synaptic spines (red) being contacted by brain-resident microglia cell (blue) - such interaction can turn destructive in multiple sclerosis.
Multiple sclerosis (MS) is a chronic inflammatory disease that affects the central nervous system, in which nerve cells are attacked by the patient’s own immune system. In many cases, the disease develops into a progressive form, which is characterized by a shift of pathology from the white matter to the grey matter, for instance to the cerebral cortex. This phase of the disease has so far been difficult to treat and its underlying causes are poorly understood. Now, a research team from the Ludwig-Maximilians Universität Mûnchen (LMU), in cooperation with the Technical University of Munich and the University of Geneva, has shown in a mouse model that inflammation of the grey matter leads to a decrease in nerve-cell activity, owing to the (potentially reversible) destruction of synapses. These results, published in the journal Nature Neuroscience, offer an interesting approach for new therapies.
Loss of synapses – the structures that serve as functional contacts between nerve cells – is an early indicator of damage to the cerebral cortex in cases of progressive MS. The researchers therefore suspected that the synapses are the key to the neuronal damage that ensues in this stage of the disease. With the aid of various imaging techniques, the team was able to demonstrate that such widespread loss of synapses can be reproduced in a mouse model of MS. Moreover, their observations revealed that synaptic spines are destroyed by a specific type of immune cells. “These immune cells preferentially eliminate spines, which contain high levels of calcium. We assume that the inflammation reaction itself triggers an influx of calcium, which destabilizes the spines,” says Martin Kerschensteiner, Director of the Institute for Clinical Neuroimmunology at LMU. “These changes in late-stage MS are reminiscent of those that can also be observed during the early phases of neurodegeneration,” Thomas Misgeld (Technical University of Munich) adds.
The disruption of neuronal networks in the brain can be reversible
The activated immune cells primarily attack excitatory synapses, which are responsible for activating other nerve cells. As a consequence, the level of activity in neural networks decreases. “The nerve cells are effectively silenced,” says Kerschensteiner. “However, much to our surprise, we discovered that this process is reversible in our model.”
As soon as the inflammation is resolved, the normal number of synapses is restored and the neurons once again exhibit their normal patterns of activity. These results contrast with findings in patients with progressive MS, in whom the cerebral cortex is permanently damaged. “Presumably, the mechanisms responsible for recovery cannot come into effect in these patients, because the inflammation is chronic and remains unresolved,” says neuropathologist Doron Merkler, Professor at the Department of Pathology and Immunology of the Faculty of Medicine of the UNIGE and Associate Associate Physician at the Clinical Pathology Service of the University Hospitals of Geneva (HUG). “In our model, we induced an acute inflammatory reaction, which is resolved within a few days.”
New pharmaceutical agents might be able to specifically inhibit the activation of the immune cells responsible for synapse destruction and could therefore slow the progress of the disease. However, it is crucial that such inhibitors do not completely block the action of these cells, so that they can continue to carry out their essential functions. The authors of the study hope that this concept can help develop therapeutic approaches that will effectively curb the progression of MS.
25 Jan 2021
