Physics and biology explore together the mechanisms of life
UNIGE researchers created a model to disentangle how proteins are unevenly distributed in cells, a process at the very basis of the development of living beings.
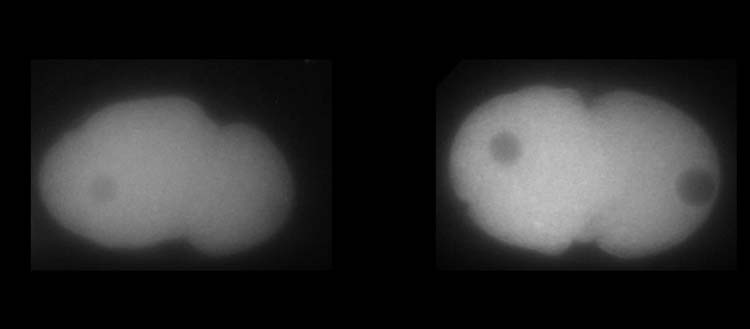
Above, MEX-5 protein. Below, PLK-1 protein in C. elegans embryos. White shows the higher or lower presence of the proteins.
© UNIGE – Laboratoire Monica Gotta
Each of our cells contains about 40 million proteins that together perform all the tasks the cell needs to survive. For a smooth action, the right proteins must be concentrated in specific amounts, at a specific time and at a specific location. However, establishing such a delicate distribution requires an extremely precise process, happening at tiny spatial resolutions that standard cell biology tools are often unable to detect. To understand how this mechanism works, researchers from the University of Geneva (UNIGE) developed a new approach combining genetics and cell biology experiments with physical modelling. Using specific algorithms, they simulated the formation of protein gradients in 3D and throughout time and were able to explain these complex mechanisms. Moreover, their innovative model can be adapted to other biology systems to investigate protein dynamics. These results can be read in the Proceedings of the National Academy of Sciences.
Like a drop of ink in a glass of water, proteins can diffuse and evenly distribute throughout the cell. However, for quite a few tasks, proteins need to form gradients. “Protein gradients, which arise from the uneven distribution of proteins in specific cellular areas, are central to many cellular and organismal functions”, explains Monica Gotta, a professor in the Department of Cell Physiology and Metabolism and in the Translational Research Centre in Onco-hematology (CRTOH) at UNIGE Faculty of Medicine, who directed this work. “For example, protein gradients are important for cell differentiation, the process by which the different cell types that constitute a complex organism emerge from a unique cell, the fertilised egg.”
A use of randomness
The PLK-1 protein, a key regulator of cell division, is known to be more concentrated at the anterior side of the embryo. But how can this mechanism be put in place, and what would be the consequence if the tiniest detail went awry? As the usual tools of biology were not sufficient to answer this question, Monica Gotta was happy to welcome in her team a physicist, Sofia Barbieri, post-doctoral researcher in the Department of Cell Physiology and Metabolism at UNIGE Faculty of Medicine. “Compiling all the known about this biological process and new hypotheses on the mechanisms, I developed a statistical model of protein gradient formation based on probabilistic mathematics“, explains Sofia Barbieri. “I resorted to specific computational algorithms, called Monte-Carlo simulations, named after the famous gambling city.” These algorithms are used to model phenomena with a high level of complexity, such as finance, trading, or particle physics.
The team was able to simulate protein gradients, not only in 3D, but also through time. Such a model required however several iterations between parameter optimisation and comparison with biological data. The researchers built a first version of the model incorporating all known physical and biological elements of the system, then introduced specific parameters necessary to test several hypotheses concerning the unknown variables. They simulated possible physical and biological outcomes that computationally reproduced the protein dynamics and gradient establishment in the cell, and tested them in real life with in vivo experiments using the embryos of a small worm, the C. elegans nematode (video).
Intricate protein interactions at play
Thanks to the continuous interplay between modelling and cell biology, the UNIGE researchers were able to determine how the PLK-1 gradient was established and maintained over time. Indeed, PLK-1 must dynamically bind to and unbind from MEX-5, another protein crucial for development in the C. elegans embryo, to counteract its natural tendency to diffuse homogenously in the cell. MEX-5 has indeed the ability to change its diffusivity depending on its position within the cell and to interact with other proteins, which is essential to enrich PLK-1 where needed. “But quite surprisingly, MEX-5 is not that efficient at its task, as a large amount of PLK-1 is not bound to MEX-5!” points out Sofia Barbieri.
This study provides a unique quantitative model for understanding dynamic interactions between proteins and can be adapted to other cells or proteins for which the complex mechanisms cannot be tested with usual cell biology experiments. “Our work shows that interdisciplinary collaborations are more and more important to advance in research!” concludes Monica Gotta.
7 Mar 2022