When healthy cells stimulate the migration of tumor cells
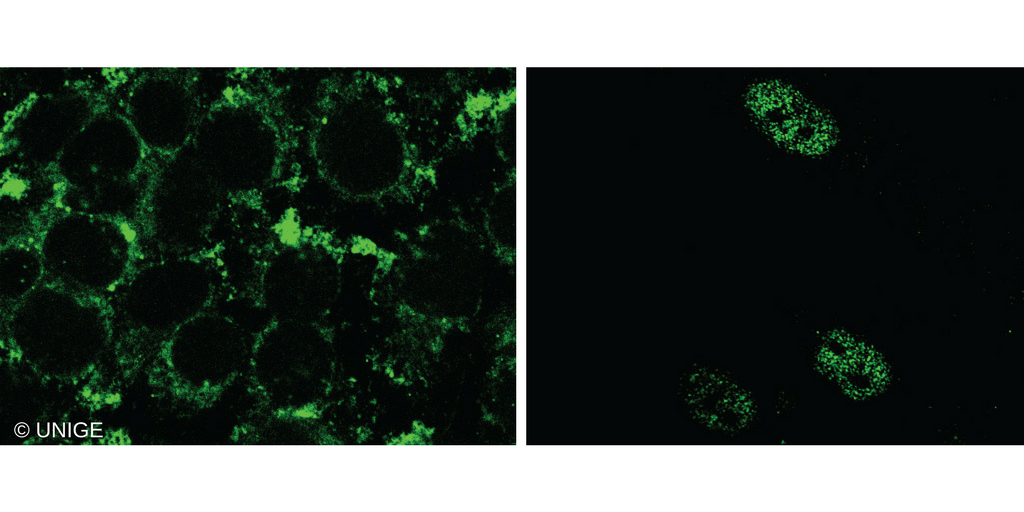
Left: cells carrying the traditional GPER (fluorescent cytoplasm). Right: cells carrying the nuclear variant of GPER (fluorescent nucleus). © UNIGE
Estrogens act as a driving force of both healthy and cancerous mammary cell growth by binding to receptors that include a type named GPER, which is generally located in cell membranes. Recent studies have, however, revealed the unusual presence of this receptor in the nuclei of fibroblasts - cells of the connective tissue - surrounding mammary tumor cells. Researchers at the University of Geneva (UNIGE), Switzerland, have discovered that this is in fact another version of GPER, a nuclear variant of this receptor, with different properties. The fibroblasts carrying this variant promote the migration of neighboring malignant cells, thus participating in the process of tumor metastasis. This research, which may pave the way to a novel therapeutic strategy, is published in the journal Oncotarget.
Estrogens play a crucial role in the genesis of the majority of breast cancers, promoting the survival, proliferation and invasiveness of tumor cells. These hormones act by binding to their receptors, which fall into two groups, ER and GPER, whose properties differ.
The team of Didier Picard, professor at the Department of Cell Biology of the Faculty of Sciences of UNIGE, is interested in GPER receptors, whose effects on breast cancer are less well known than those of the ER family. “By analyzing a breast tumor biopsy, our colleagues at the University of Calabria, who participated in this study, discovered GPER receptors in the nucleus of fibroblasts present around the malignant cells, whereas they had always been localized elsewhere before”, says the biologist.
Fibroblasts abound around the tumor
Fibroblasts, constituents of the connective tissue, are part of the microenvironment of the tumors. “Knowing that cancer cells interact with neighboring healthy cells to survive and grow, we wanted to find out whether the GPER receptors observed in the nucleus of these fibroblasts were involved in this process”, explains Marco Pupo, first author of the study.
The researchers have discovered the existence of another version of GPER: “This genetic variant results from a tiny change, a single nucleotide, in the gene that codes for the GPER receptor. This is sufficient to induce its relocation to the nucleus. We detected the presence of this same version of the gene in the fibroblasts of the eight other biopsies we analyzed, from the University Hospital of Geneva”, states Didier Picard.
The genetic variant of GPER could predispose to breast cancer
The nuclear variant also acquires new properties. Indeed, unlike the GPER receptor hitherto described, it is capable of stimulating the expression of genes involved in malignant growth. Moreover, fibroblasts present in the tumor microenvironment and carrying nuclear GPER secrete molecules that promote the migration of neighboring breast cancer cells.
“We have deciphered an important element of the dialogue between the malignant cells and the adjacent healthy cells, and we want to find out the nature of the fibroblastic factors involved in the process of tumor metastasis”, specifies Marco Pupo. It may be worth thinking about specifically interfering with the nuclear functions of GPER as a novel therapeutic strategy.
Contact: Didier Picard, +41 22 379 68 13
15 Jun 2017