The above described approaches allow us to investigate several interesting aspects of biologically relevant interfaces. Our main aim is to increase our understanding on the relation between the properties of aqueous solutions and the interfaces. For example, how does the pH of the interface depend on the pH of the aqueous solution? Absorption of most photoacids is strongly dependent on pH and therefore these compounds can be used as interfacial pH-probes as illustrated in Figure 3. With phospholipid membranes, we can additionally investigate the influence of different phospholipid head groups (e.g. neutral, cationic, or anionic). Comparison with control measurements in bulk solutions allows us to address problems related to structure water, solvation dynamics, ESPT and diffusion of protons at these interfaces. Due to the thorough understanding of the influence of the environment on ESPT established in bulk solutions, the properties of the interface-bound photoacids are expected to directly report on the local environment at interfaces.
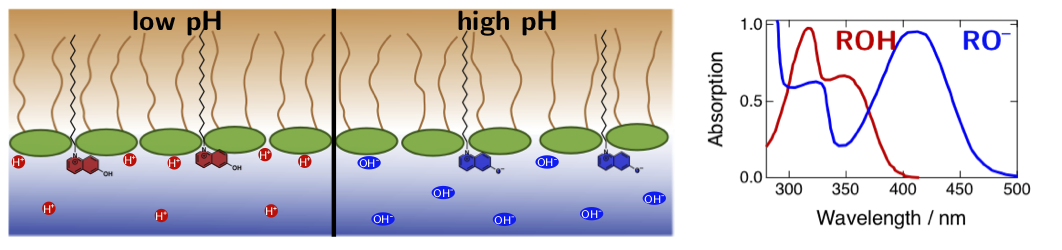 Figure 3. Absorption spectra of photoacids depends strongly on pH due the equilibrium between the acid (ROH) and the base (RO–) forms. Therefore, these compounds can be additionally used as pH-sensitive probes for biological interfaces.
Figure 3. Absorption spectra of photoacids depends strongly on pH due the equilibrium between the acid (ROH) and the base (RO–) forms. Therefore, these compounds can be additionally used as pH-sensitive probes for biological interfaces.
proton transfer at biological interfaces
Proton transfer in biological processes often occurs at the interface between aqueous and hydrophobic media, such as the surfaces of proteins and cell membranes, hence being distinct from processes in solutions. Several interesting hypotheses about ESPT, proton diffusion and proton concentration at interfaces have been put forward but direct unambiguous experimental evidence is scarce. Some research suggests that proton diffusion can occur along these surfaces, which is of great importance for cellular respiratory processes. Secondly, air–water interfaces have been shown to be significantly more acidic than the bulk aqueous media but the relation between the bulk acidity and the interfacial acidity is not well understood. Increased affinity of protons and other ions towards such interfaces can have an impact on several environmental and atmospheric chemical reactions, which often occur on the surfaces of water droplets, lakes and oceans.
We want to utilize photoacids in combination with spectroscopic techniques to explore the ESPT reactions first at model interfaces between water and non-polar organic solvents, and then move towards biologically relevant interfaces such as phospholipid membranes. The main challenge in investigating interfacial processes is to discriminate the signal from the bulk material from that of the interfacial region. To overcome this problem, we use an interface-specific technique, surface Second Harmonic Generation (SSHG), which can yield valuable information about the orientation, spectral properties, and dynamics of the ESPT process at the interfaces. SSHG is based on a nonlinear optical process that is electric dipole forbidden in centrosymmetric or isotropic media such as bulk solutions. Therefore, the signal is generated only from the very narrow interfacial region without contributions from the bulk media. The photoacids can be either adsorbed to the interface from the aqueous layer or be directly transferred to the interface before adding the top layer. Our experimental approach for a model interface between water and dodecane is depicted in Figure 1. We use specially functionalized photoacids that have aliphatic tails to increase the affinity of the compounds towards interfaces between aqueous and apolar media. Such compounds can be additionally used as pH-probes to investigate the acidity (proton concentration) at these interfaces.
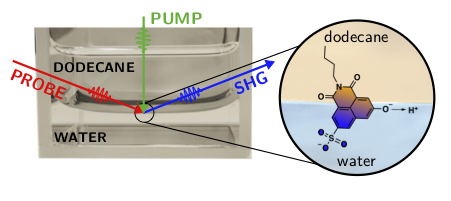
Figure 1. Specially designed photoacid is placed at the interface between aqueous and hydrophobic media. The probe beam will generate the SHG signal only from the interface-adsorbed photoacids. Pump laser is used to initiate the ESPT, which is then followed as a function of time.
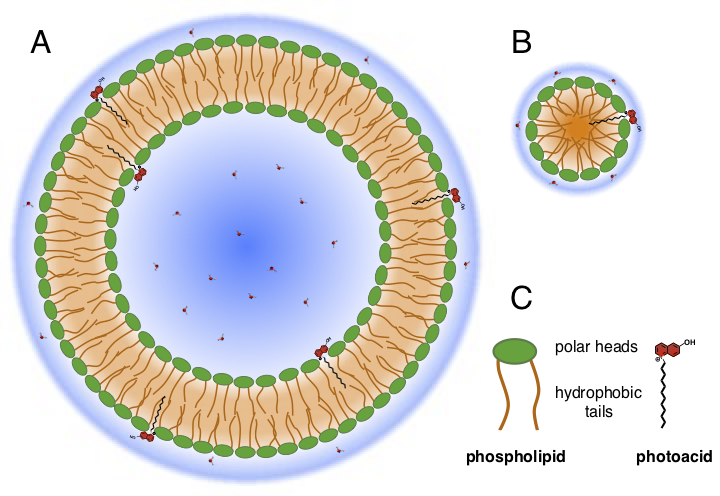
Figure 2. Schematic representations of photoacid-decorated phospholipid liposomes (A) and micelles (B).
Another approach is based on photoacid-decorated phospholipid micelles and liposomes. The main advantage of this approach is that micelles and liposomes can be easily dispersed into aqueous solutions, while the photoacids remain bound to the interface (Figure 2). Therefore, we can use conventional spectroscopic techniques such as steady-state and time-resolved absorption and fluorescence to investigate the properties of the photoacids at these interfaces. Conventional time-resolved techniques enable investigations on much longer time scales up to milliseconds whereas the time-resolved SSHG is limited to ca. 1–1.5 ns. This is perfectly suitable for investigating the dissociation process, but the geminate recombination usually takes place on a much longer time scale, out of the reach of the SSHG technique. The diameter of the micelles and liposomes can be tuned from a few nm (micelles) up to 100–200 nm (liposomes) by changing the sample preparation conditions or the surfactant. If the dissociated proton is diffusing on the phospholipid surface without dissociation into the bulk, recombination is expected strongly dependent on the surface area. Thus, studies on the recombination kinetics as a function of the size of the micelles and liposomes could potentially reveal some key insights about the proton diffusion at the interfaces.
