solvation dynamics
Polar solvation dynamics play a key role in several solution phase chemical reactions, especially those involving charged or dipolar species, such as electron-transfer and proton-transfer reactions. Interaction with the polar environment can alter the reaction barrier through different stabilization of the reactant, transition or product states thus directly influencing the reaction rate. Moreover, investigations of the solvation dynamics on complex chemical or biological environments can yield valuable information about the local dielectric surroundings that is otherwise difficult or even impossible to access. For all the above cases, detailed understanding of the solvation dynamics and their dependence on the probe and the solvent is of paramount importance.
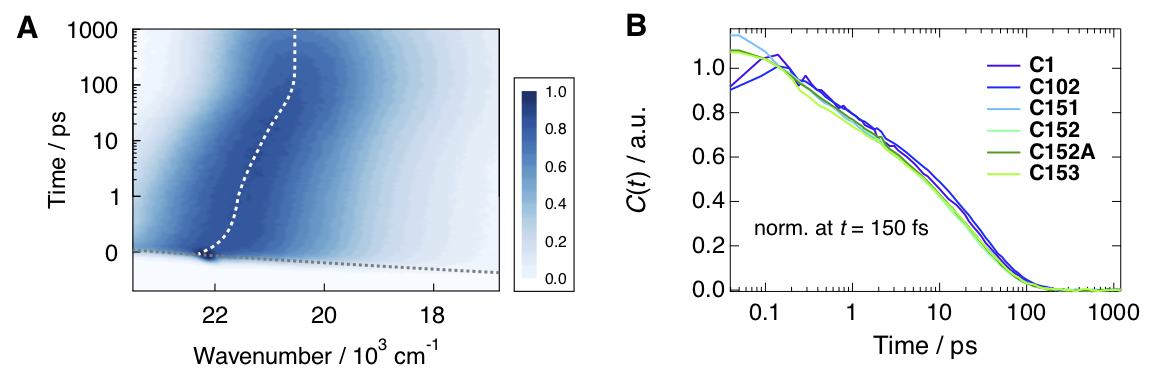
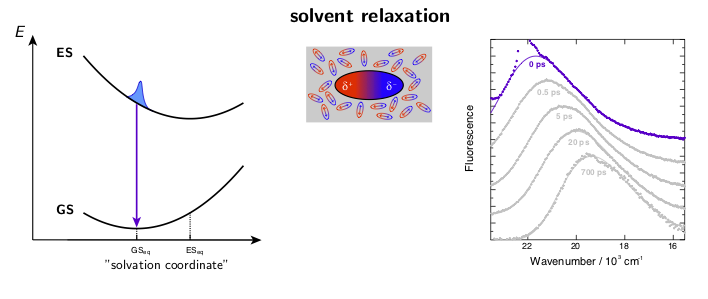
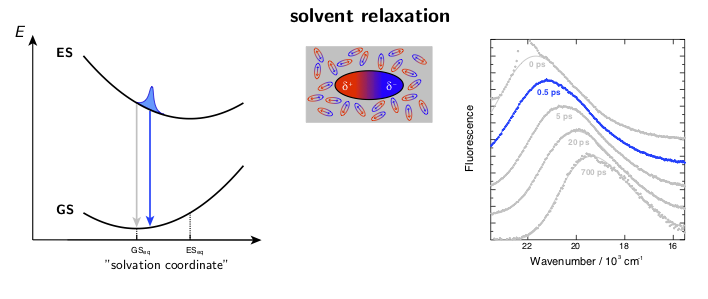
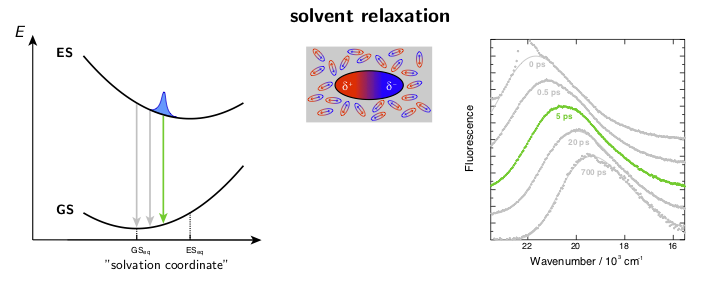
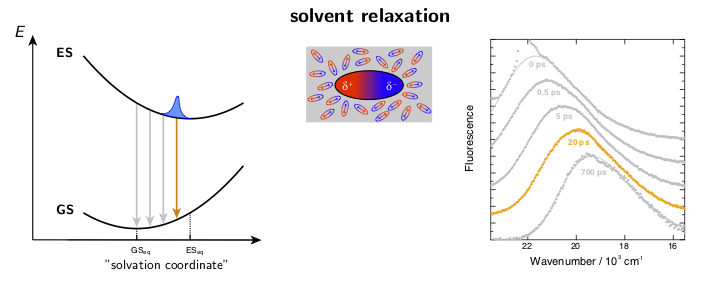
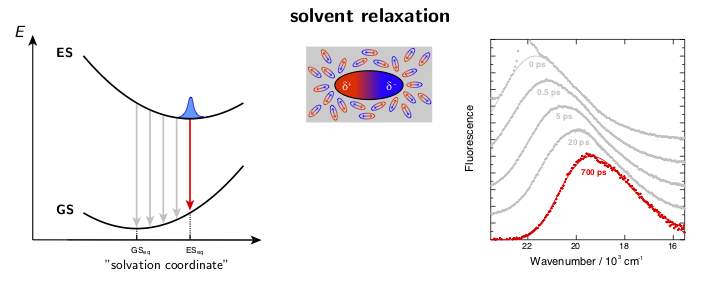
Figure 1. A) Example broadband fluorescence spectra of coumarin 151 (C151) in ethanol. The white dashed line shows the time evolution of the fluorescence band position. B) Normalized spectral relaxation functions (eqn. 1) for a set of coumarins in ethanol.
Solvation dynamics can be investigated using several approaches, the time-dependent Stokes shift method being one the most used due to its simplicity. This method utilizes push–pull type fluorophores such as coumarins, which exhibit a large difference in the permanent dipole moment between the ground and the excited states. Upon excitation of such push-pull molecules, the electric field generated by the solute dipole is suddenly changed on a time scale that is too fast for the solvent to respond. Therefore, the surrounding solvent begins to reorient to accommodate the new charge distribution in order to minimize the solvation free energy. During this process, the free energy separation of the ground and excited states is reduced resulting in the dynamic Stokes shift (frequency down-shift) of the fluorescence emission. The spectral response function, C(t), a measure of the solvation free energy, is obtained from the time evolution of the fluorescence band position as:

where ν(0), ν(t), and ν(∞) are the frequencies of the fluorescence band position at time 0, t, and ∞, respectively. Example fluorescence data together with the spectral relaxation functions are presented in Figure 1.
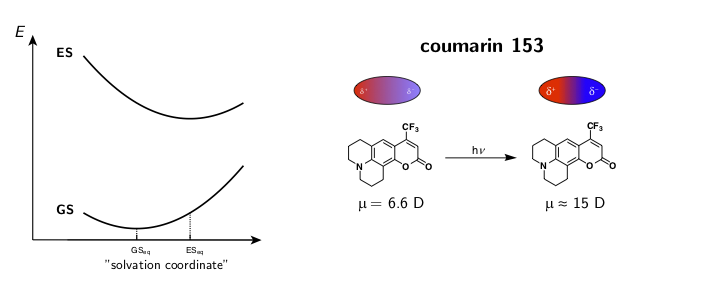
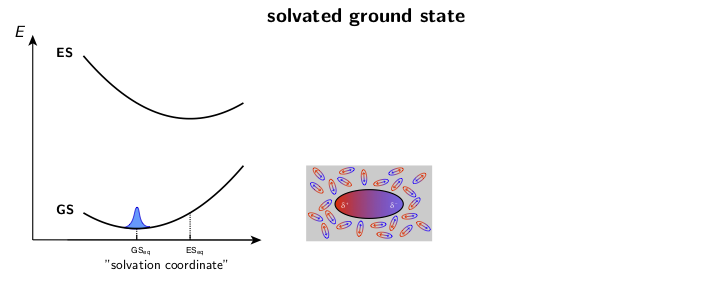
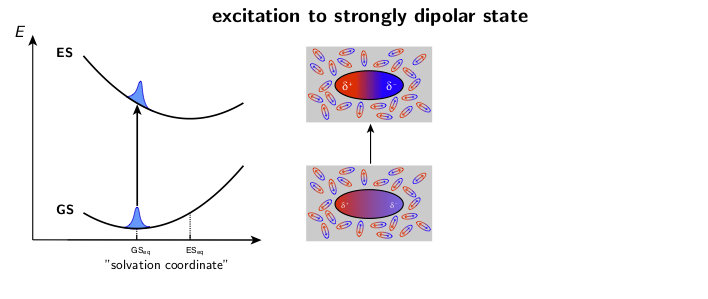
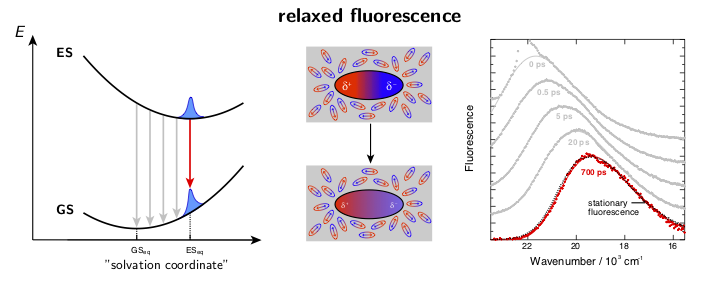
The dynamic solvent relaxation together with the time-dependent Stokes shift using coumarin 153 (C153) as an example are schematically illustrated below. C153 is weakly dipolar in its ground state (GS) and strongly dipolar in its first excited state (ES). Two potential energy surfaces corresponding to the ground and excited states are plotted as a function of so-called “solvent coordinate” (left part of the animation). In the ground state, the solvent molecules are relatively randomly oriented around the weakly dipolar solute . Upon excitation, C153 is instantaneously switched to the strongly dipolar state while the solvent remains in the ground-state equilibrium configuration. At this point, the fluorescence exhibits only a relatively small Stokes shift. The energy gap between the ground and excited states is reduced during the solvent reorientation resulting in time-dependent Stokes shift of the fluorescence band (right part of the animation). After reaching the fully relaxed excited-state solvent configuration, the fluorescence nearly coincides with the steady-state fluorescence measured on a standard spectrofluorometer.
We are interested in several fundamental aspects of the solvent relaxation. For example, how does the chemical structure of the solute influence the solvent relaxation in the same solvent or how does the structure of the solvent influence the relaxation of the same solute. Moreover, detailed investigations allow to test theoretical predictions such as different solvatochromic models based on linear response theories. We are also working on developing novel methods for a global analysis of the broadband fluorescence spectra. Besides the dynamic solvent relaxation, we also investigate static solvatochromism using standard steady-state spectroscopy.