Liste
Précédente Suivante
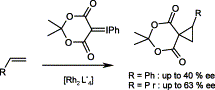
Publication 166
-
P. Müller, Y. Allenbach, E. Robert,
“Rhodium(II)-catalyzed olefin cyclopropanation with the phenyliodonium ylide derived from Meldrum's acid”
Tetrahedron: Asymmetry 2003, 14, 779-785.
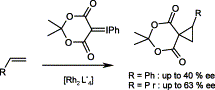
The phenyliodonium ylide 3a derived from Meldrum's acid reacts with olefins in the presence of Rh(II) carboxylate catalysts to afford cyclopropanes. The reaction is stereospecific. Enantioselectivities of up to 63% have been observed for the cyclopropanation of pent-1-ene. No 1,3-cycloadducts are formed between 3a and polarized olefins such as furan or 2,3-dihydrofuran.
