Liste
Précédente
Publication 213
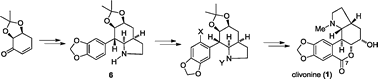
- Total synthesis of the Amaryllidaceae alkaloid clivonine
Haning, H.; Giró-Mañas, C.; Paddock, V. L.; Bochet, C. G.; White, A. J. P.; Bernardinelli, G.; Mann, I.; Oppolzer, W.; Spivey, A. C.
Org. Biomol. Chem. 2011, 9, 2809-2820

Two syntheses of the Amaryllidaceae alkaloid clivonine (1) are described. Both employ previously reported 7-arylhydrindane 6 as an intermediate but differ in the method employed for subsequent introduction of what becomes the ring-B lactone carbonyl carbon (C7). The synthesis featuring a Bischler–Napieralski reaction for this transformation constitutes the first asymmetric synthesis of natural (+)-clivonine. Crystal structures for compounds (±)-13, (±)-16, (−)-20 and (±)-28 are also reported.
DOI : 10.1039/c0ob00895h
archive ouverte unige:155655
