Liste
Précédente Suivante
Publication 193
- Polycyclic Indoline-Benzodiazepines by Electrophilic Additions of α-Imino Carbenes to Tröger Bases
Alessandro Bosmani, Alejandro Guarnieri-Ibáñez, Sébastien Goudedranche, Céline Besnard, Jérôme Lacour
Angew. Chem. Int. Ed. 2018, 57, 7151-7155

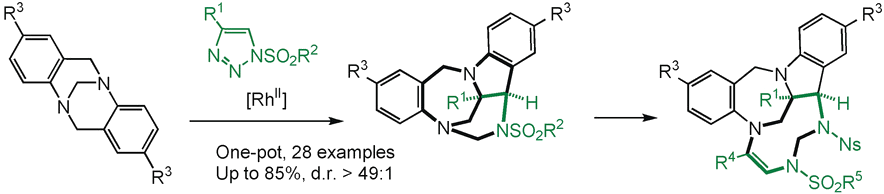
Polycyclic indoline-benzodiazepines are afforded by the intermolecular reaction of Tröger bases with N-sulfonyl-1,2,3-triazoles. Under Rh(II)-catalysis, α-imino carbenes are generated and a subsequent cascade of [1,2]-Stevens, Friedel-Crafts, Grob and aminal formation reactions yield the polycyclic heterocycles as single isomers (d.r.>49:1, four stereocenters incl. two bridgehead N-atoms). Further ring-expansion by insertion of a second α-imino carbene leads to elaborated polycyclic 9-membered ring triazonanes.
DOI : 10.1002/anie.201803756
archive ouverte unige:105328
