Publication 201
- Circularly Polarized-Electrochemiluminescence from a Chiral Bispyrene Organic Macrocycle
Francesco Zinna, Silvia Voci, Lorenzo Arrico, Elodie Brun, Alexandre Homberg, Laurent Bouffier, Tiziana Funaioli, Jérôme Lacour, Neso Sojic, Lorenzo Di Bari
Angew. Chem. Int. Ed. 2019, 58, 6952-6956
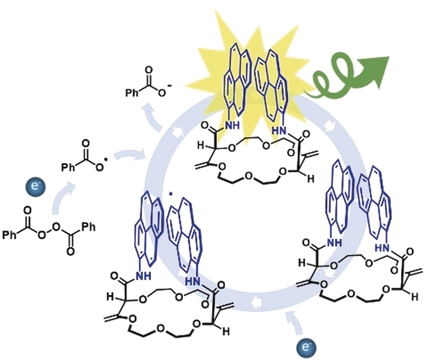
The first observation of circular polarization of electrochemiluminescence (ECL) from a purely organic derivative is reported. A bis-pyrene scaffold mounted on a constrained polyether macrocycle displaying intense excimer fluorescence and highly circularly polarized (CP) photoluminescence has been selected for this purpose. Such compound displays an ECL dissymmetry factor of ca. |8×10-3|, in good agreement with the corresponding photoluminescence value. This observation is the first step towards the molecular engineering of bespoke suitable dyes acting both as ECL and CP-ECL reporters for (bio)analysis by bringing a new level of information when dealing with chiral environments. In addition, it provides an extra-dimension to the ECL phenomenon and opens the way to chiral detection and discrimination.
DOI : 10.1002/anie.201901303
archive ouverte unige:117369
