Liste
Précédente Suivante
Publication 207
- Enantiopure encaged Verkade's superbases: Synthesis, chiroptical properties, and use as chiral derivatizing agent
Jian Yang, Bastien Chatelet, Damien Hérault, Véronique Dufaud, Vincent Robert, Stéphane Grass, Jérôme Lacour, Nicolas Vanthuyne, Marion Jean, Muriel Albalat, Jean-Pierre Dutasta, Alexandre Martinez
Chirality 2020, 32, 139-146

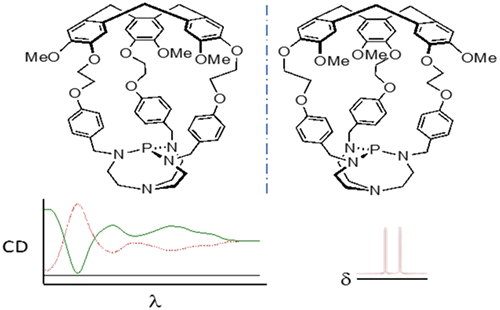
Verkade's superbases, entrapped in the cavity of enantiopure hemicryptophane cages, have been synthesized with enantiomeric excess (ee) superior to 98%. Their absolute configuration has been determined by using electronic circular dichroism (ECD) spectroscopy. These enantiopure encaged superbases turned out to be efficient chiral derivatizing agents for chiral azides, underlining that the chirality of the cycloveratrylene (CTV) macrocycle induces different magnetic and chemical environments around the phosphazide functions.
Supporting Information (PDF / 2 MB)
DOI : 10.1002/chir.23156
archive ouverte unige:129075
