Liste
Précédente Suivante
Publication 191
-
R. Herrero, E. Quintanilla, P. Müller, J.-L.M. Abboud,
“The relationship between thermodynamic stabilities of carbenium ions in the gas phase and in aqueous solution: A wide scope study”
Chem. Phys. Lett. 2006, 420, 493-496.
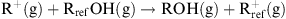
The thermodynamics of the reaction involving Rref = tert-butyl and 21 carbenium ion/alcohol systems has been studied at the MP2/6-311+G(3d, 2p)//MP2/6-311+G(d, p) level. The results have been compared to the experimental data for the same systems in solution. The range of structural effects spans 102 kcal mol-1 in the gas phase and is the widest ever examined. A physically meaningful correlation has been found. Phenyl-substituted systems are among the most important exceptions.
