Intérêts de recherche (en anglais)
Asymmetric Catalysis in Transfer of Carbenes and Nitrenes, and in Nucleophilic Substitutions
The objective of the research consists in the conversion of achiral or racemic molecules into chiral products by means of appropriate catalysts.
The main domains of interest are:
- Transition metal-catalysed decomposition of diazo compounds
- Generation and enantioselective transfer of metal carbenoids from phenyliodonium ylides
- Enantioselective transfer of nitrenes
- Desymmetrisation of aziridines with chiral nucleophiles and bases
- Enantioselective nucleophilic substitution at prochiral, sp3-hybridized carbon
Transition metal-catalysed decomposition of diazo compounds
The decomposition of diazo compounds by Rh(II)- or Cu(I)-complexes affords metal carbenoids. These intermediates exhibit selectivities different from that of free carbenes. If the metal carries appropiate ligands, enantioselective carbene transfer may occur. Representative examples are given below:
- Intramolecular cyclopropanation:
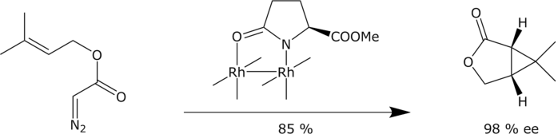
M. P. Doyle, R. J. Pieters, S. F. Martin, R. E. Austin, C. J. Oalmann, P. Müller,
J. Am. Chem. Soc. 1991, 113, 1423-1424.
- Intermolecular cyclopropenation:
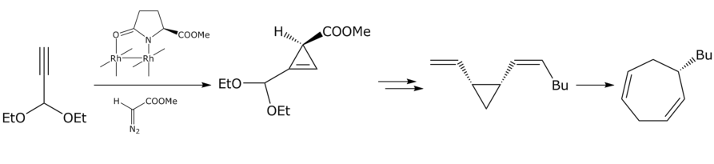
H. Imogai, G. Bernardinelli, C. Gränicher, M. Moran, J.-C. Rossier, P. Müller,
Helv. Chim. Acta. 1998, 81, 1754-1764.
- Intramolecular CH insertion:

P. Müller, P. Polleux,
Helv. Chim. Acta 1994, 77, 645-654.
Generation and enantioselective transfert of metal carbenoids from phenyliodonium ylides
We are investigating the possible use of phenyliodoniumylides as substitutes for diazo compounds in carbenoid reactions. The Rh(II)-catalysed intramolecular CH insertion of diazoester 1a below proceeds with the same enantioselectivity as the corresponding phenyliodonium ylide 1b.
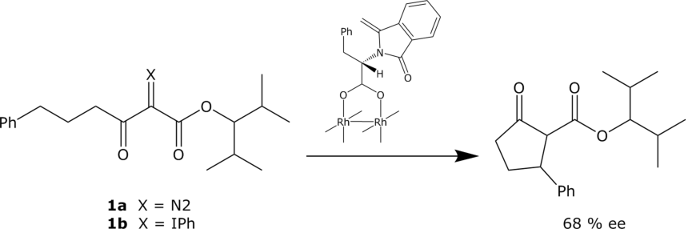
Similarily, enantioselective intramolecular cyclopropanations and CH insertions may be realized upon decomposition of phenyliodonium ylides with chiral Cu-catalysts. The Cu-catalysed intramolecular insertion of phenyliodonium ylides is stereospecific and proceeds with retention of configuration.
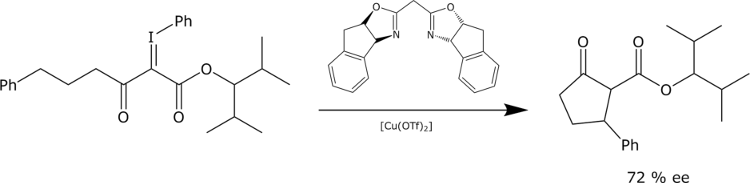
P. Müller, D. Fernandez,
Helv. Chem. Acta. 1995, 78, 947-958.
P. Müller, C. Boléa,
Synlett 2000, 826-828.
P. Müller, C. Boléa,
Molecules 2001, 6, 258.
Enantioselective transfer of nitrenes
The decomposition of phenyliodonium ylides derived from aromatic sulfonamides in the presence of chiral copper catalysts results in aziridination. We have realized the same reaction with chiral Rh(II) catalysts. The Rh(II)-catalysedaziridination is stereospecific. So far, only modest enantioselectivities have been achieved. The metal nitrenes obtained upon deconposition of these phenyliodinanes may also undergo insertions into CH bonds. The reaction is stereospecific and proceeds with retention of configuration at the reacting carbon atom.
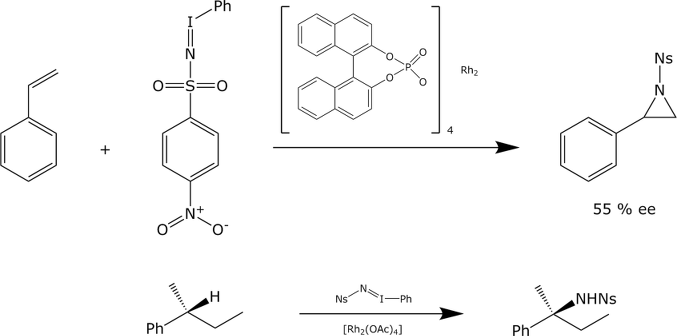
P. Müller, C. Baud, I. Nägeli,
J. Phys. Org. Chem. 1998, 11, 597-601.
P. Müller, C. Baud, Y. Jacquier,
Can. J. Chem. 1998, 76, 738-750.
I. Nägeli, C. Baud, G. Bernardinelli, Y. Jacquier, M. Moran, P. Müller,
Helv. Chim. Acta 1997, 80, 1087-1105.
Desymmetrisation of aziridines with chiral nucleophiles and bases
Meso-aziridines undergo enantioselective ring-opening with Grignard reagents or organolithium compounds in the presence of chiral, non-racemic copper catalysts. With sec-butyllithium and an optically active tertiary amine such as (-)-sparteine, the aziridines may rearrange to an allylic amide or suffer an intramolecular CH insertion via a carbene.

P. Müller, P. Nury,
Org. Lett. 1999, 1, 439-441.
P. Müller, P. Nury,
Helv. Chim. Acta. 2001, 84, 662-667.
Enantioselective nucleophilic substitution at prochiral, sp3-hybridized carbon
Acetals undergo enantioselective mono-substitution by organolithium reagents in the presence of (-)-sparteine and BF3. At the present stage of development, the reaction is not catalytic, and the enantioselectivity is only moderate. However, current research is directed towards generalization and further development of enantioselective nucleophilic substitutions.

P. Müller, P. Nury,
Org. Lett. 2000, 2, 2845-2847.
P. Müller, P. Nury, G. Bernardinelli,
Eur. J. Org. Chem. 2001, 21, 4137-4147.
