- Fluorescence Quenching in Electron-Donating Solvents. 1. Influence of the Solute-Solvent Interactions on the Dynamics
Ana Morandeira, Alexandre Fürstenberg, Jean-Claude Gumy, and Eric Vauthey
J. Phys. Chem. A 2003, 107(28), 5375-5383
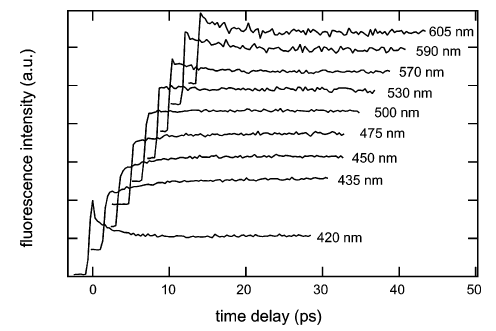 |
The electron transfer (ET) quenching dynamics of excited perylene (Pe), cyanoperylene (PeCN), methanolperylene (PeOH), and methylperylene (PeMe) in N,N-dimethylaniline (DMA) has been investigated using ultrafast fluorescence up-conversion. Measurements of the rotational dynamics of PeCN and PeMe in nonpolar and polar inert solvents using optically heterodyned polarization spectroscopy are also presented. The fluorescence decay in DMA is strongly nonexponential and about 10 times faster with PeCN than with the other electron acceptors. The quenching dynamics has been analyzed with a model distinguishing three types of donor molecules surrounding the acceptor: those with optimal orientation for ET and those requiring orientational or translational diffusion prior to ET. According to this model, which can account for the whole fluorescence decay, the faster quenching dynamics of PeCN is not due to a larger ET rate constant, but to a larger number of donor molecules, typically three to four, with an optimal orientation. This is explained by the effect of dipole−dipole interaction between PeCN and the donor molecules, which favors mutual orientations with a large electronic coupling. With the other acceptors, this interaction is either not present or does not lead to ET active geometries. The occurrence of this interaction is substantiated by the rotational dynamics measurements.
DOI: 10.1021/jp0343133
Archive ouverte / Open archive: unige:3610
