Publication 110
- Highly enantioselective biphasic iminium-catalyzed epoxidation of alkenes. On the importance of the counterion and of N(sp2)—C(sp3) rotamers
Roman Novikov, Gérald Bernardinelli, Jérôme Lacour
Adv. Synth. Catal. 2009, 351, 596-606
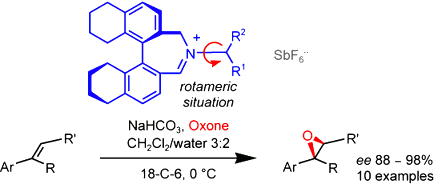
Diastereomeric biaryliminium cations made of an (Ra)-5,5’,6,6’,7,7’,8,8’-octahydrobinaphthyl core and exocyclic appendages derived from (S)- or (R)-3,3-dimethylbutan-2-amine are effective asymmetric epoxidation catalysts for unfunctionalized alkenes. Herein, we report that the negative counterion of the iminium salts has to be chosen wisely. While the hexafluoroantimonate anion [SbF6-] is optimal for reliable results, one has to be careful about other anions and tetraphenylborate [BPh4-] in particular. We also detail that the so far unexplained “lack” of stereochemical control from the chiral exocyclic appendage in this type of catalysts is due to the existence of atropisomers around the N(sp2)—C(sp3) bond that links the azepinium core to the exocyclic stereocenter. Finally, we develop a general model to predict with certainty the high selectivity in the formation of non-racemic epoxides of defined absolute configuration.
DOI : 10.1002/adsc.200800778
archive ouverte unige:7052
