Publication 149
- Chiral Selectivity in the Binding of [4]Helicene Derivatives to Double-Stranded DNA
Oksana Kel, Alexandre Fürstenberg, Nathalie Mehanna, Cyril Nicolas, Benoît Laleu, Martin Hammarson, Bo Albinsson, Jérôme Lacour, Eric Vauthey
Chem. Eur. J. 2013, 19, 7173-7180
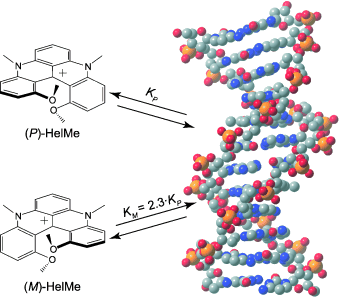
The interaction of a series of chiral cationic [4]helicene derivatives, which differ by their substituents, with double-stranded DNA has been investigated by using a combination of spectroscopic techniques, including time-resolved fluorescence, fluorescence anisotropy, and linear dichroism. Addition of DNA to helicene solutions results to a hypochromic shift of the visible absorption bands, an increase of fluorescence quantum yield and lifetime, a slowing down of fluorescence anisotropy decay, and a linear dichroism in flow-oriented DNA, which unambiguously points to the binding of these dyes to DNA. Both helicene monomers and dimeric aggregates, which form at higher concentration, bind to DNA, the former most probably upon intercalation and the latter upon groove binding. The binding constant depends substantially on the dye substituents and is, in all cases, larger with the M than the P enantiomer, by factors ranging from 1.2 to 2.3, depending on the dye.
DOI : 10.1002/chem.201203915
archive ouverte unige:27926
