Publication 54
- Expanding the Scope and Orthogonality of PNA Synthesis
S. Pothukanuri, Z. Pianowski, N. Winssinger
Eur. J. Org. Chem. 2008, 3141-3148
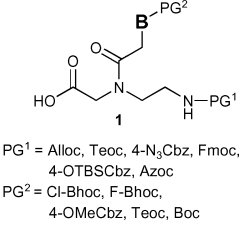
We present a thorough investigation of six types of protecting groups for the terminal nitrogen atom and five protecting groups on the nucleobases of peptide nucleic acids for fully orthogonal synthesis with Fmoc.
Peptide nucleic acids (PNAs) hybridize to natural oligonucleotides according to Watson and Crick base-pairing rules. The robustness of PNA oligomers and ease of synthesis have made them an attractive platform to encode small or macromolecules for microarraying purposes and other applications based on programmable self assembly. A cornerstone of these endeavors is the orthogonality of PNA synthesis with other chemistries. Herein, we present a thorough investigation of six types of protecting groups for the terminal nitrogen atom (Alloc, Teoc, 4-N3Cbz, Fmoc, 4-OTBSCbz, and Azoc) and five protecting groups on the nucleobases (Cl-Bhoc, F-Bhoc, Teoc, 4-OMeCbz, and Boc).
DOI : 10.1002/ejoc.200800141
archive ouverte unige:24724
